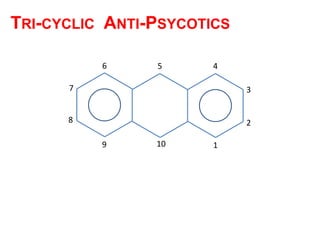
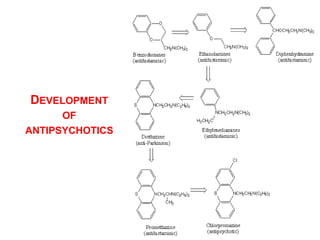
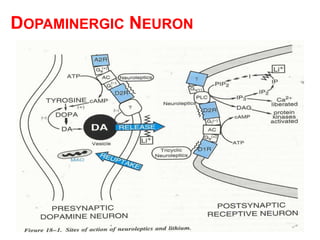
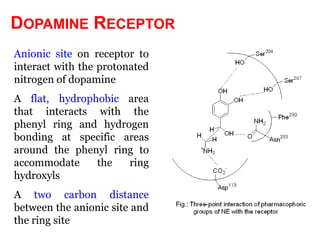
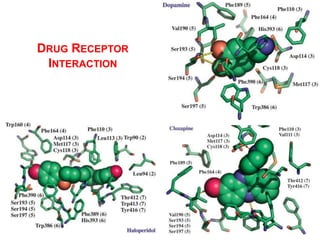
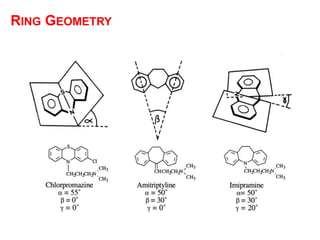
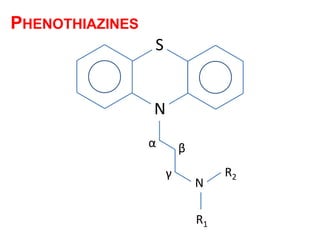
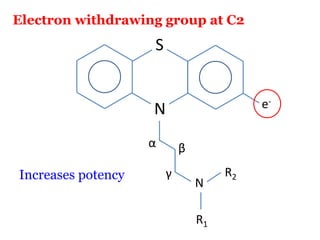
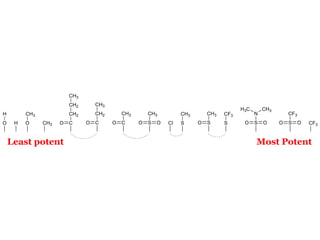
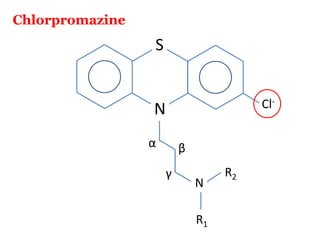
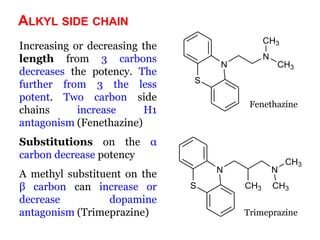
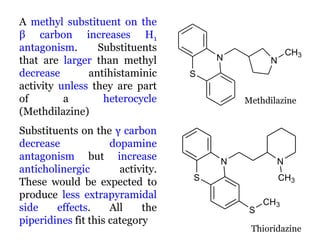
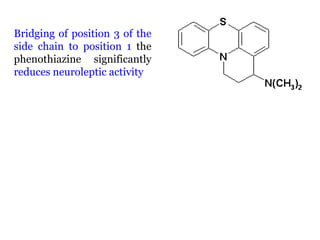
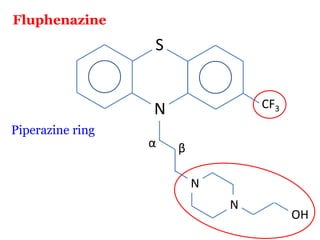
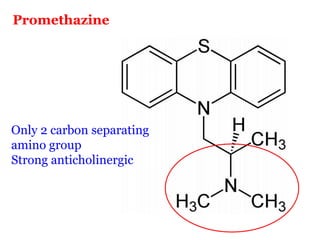
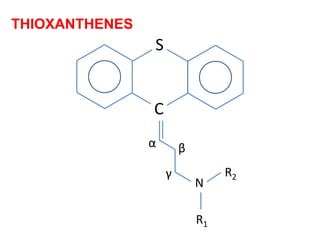
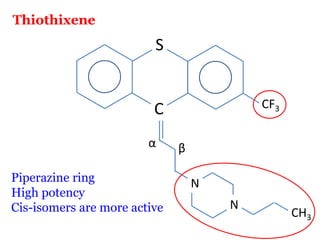
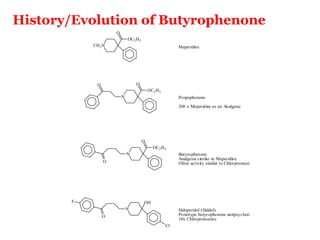
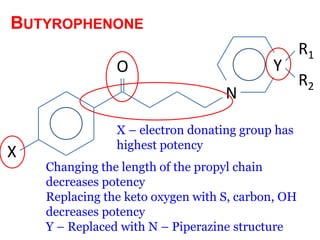
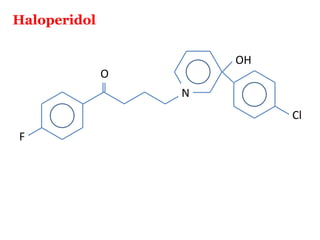
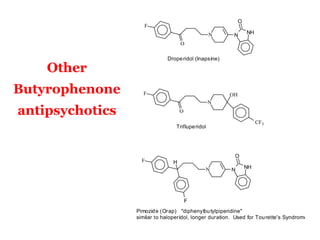
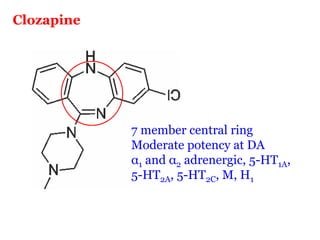
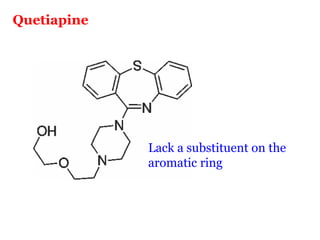
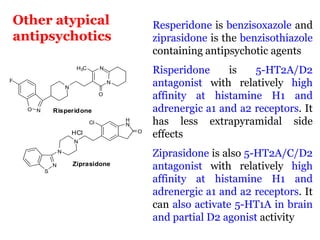
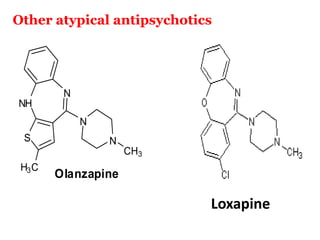
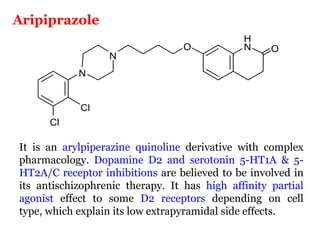
The document discusses the structure-activity relationships of various antipsychotic drugs. It begins by describing the dopamine receptor and how drugs like phenothiazines are able to bind to it. It then covers specific structural features of different drug classes that impact their potency and side effect profiles, including phenothiazines, butyrophenones, and atypical antipsychotics. Key structural attributes discussed are the presence of electron-withdrawing groups on phenothiazine rings, different side chains on phenothiazines and butyrophenones, and the chemical structures of atypical antipsychotics. The document concludes that while older structure-function concepts were important, current antipsychotics have diverse mechanisms of action involving