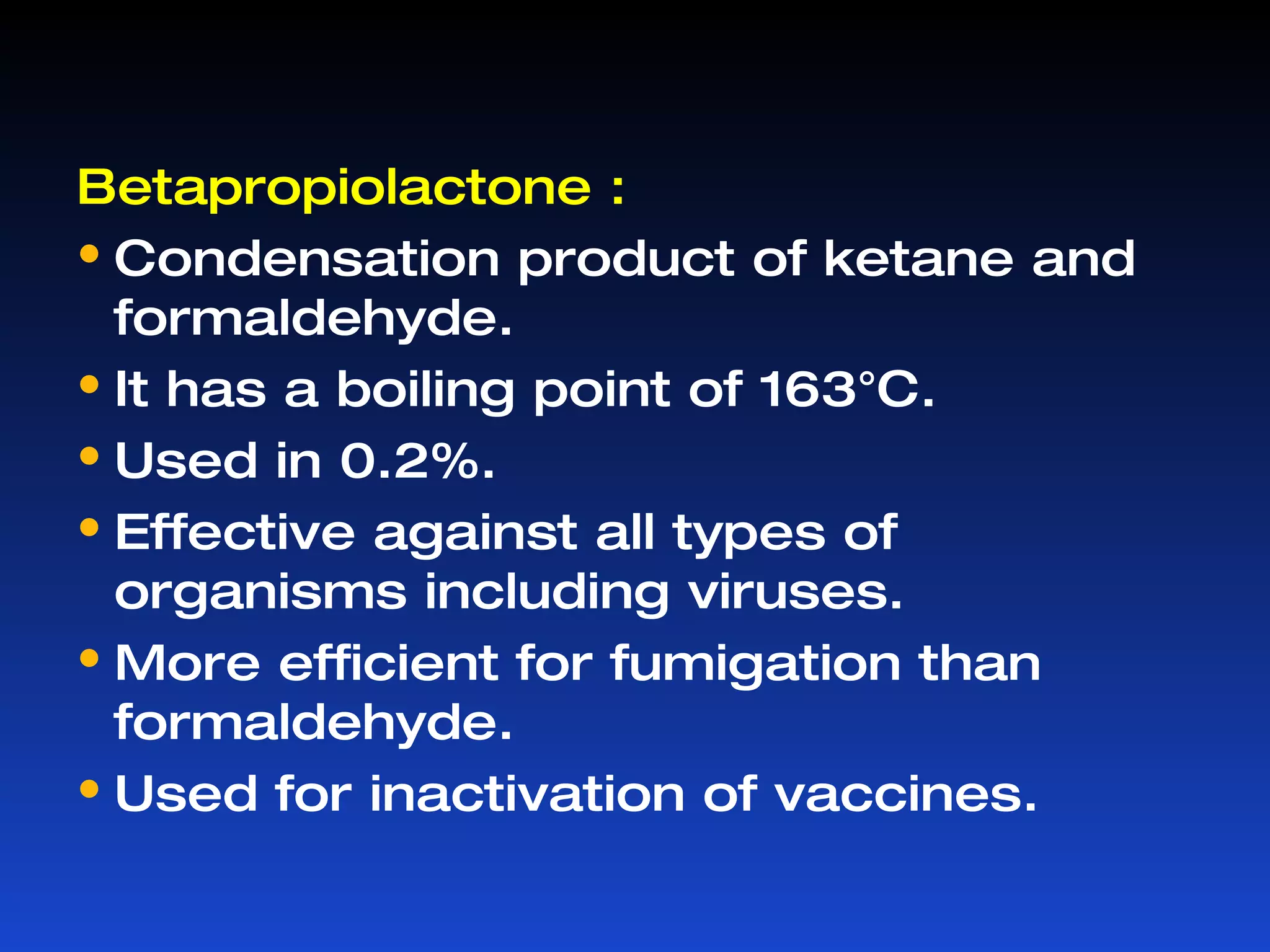
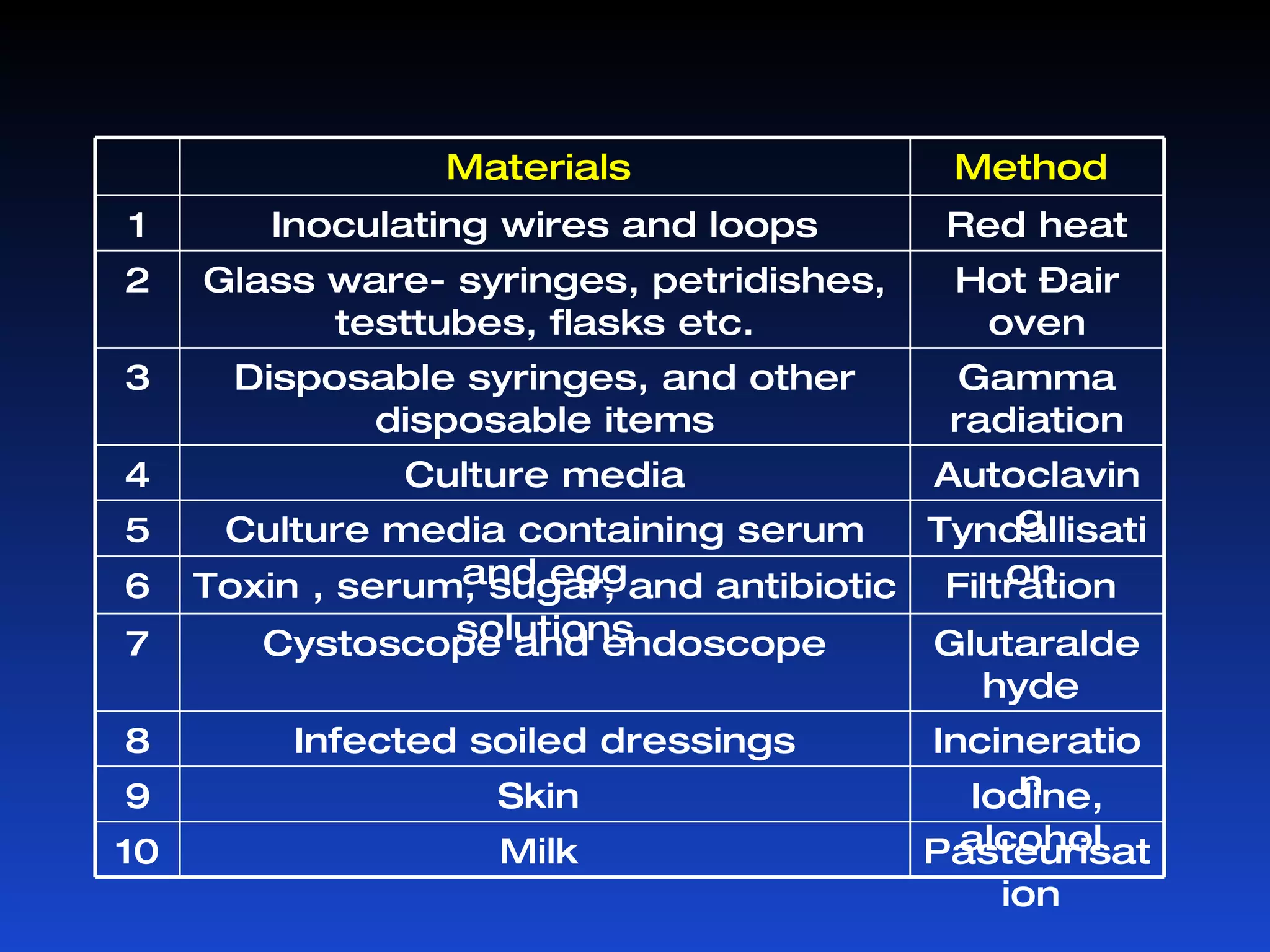
Microorganisms can cause infection and are present everywhere. Sterilization aims to remove or destroy microorganisms from surfaces and materials. Various physical and chemical methods are used for sterilization with different effectiveness against bacterial vegetative cells, spores, viruses, and other microbes. Common physical sterilization methods include heat, radiation, and filtration, while chemical methods include alcohols, aldehydes, phenols, halogens, and other disinfecting agents. The choice of sterilization method depends on the type of material or substance being sterilized.