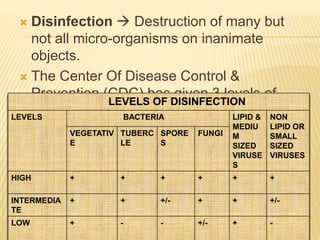
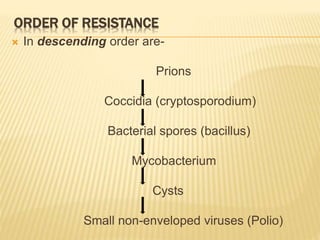
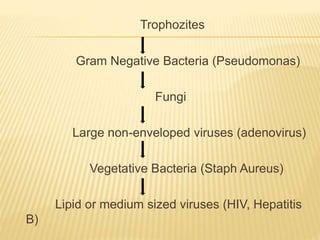
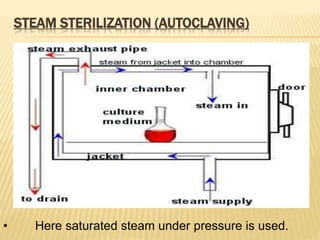
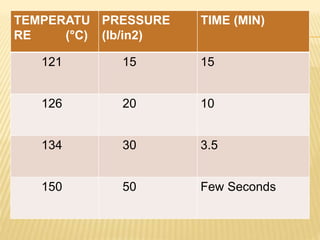
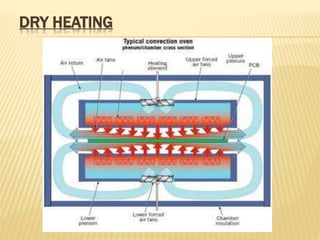
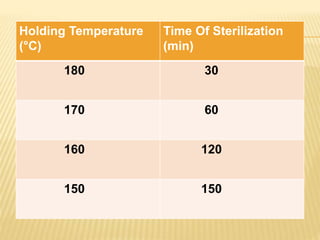
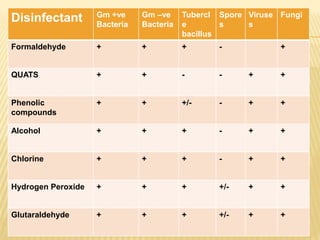
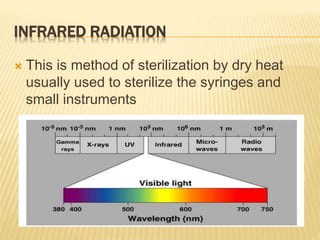
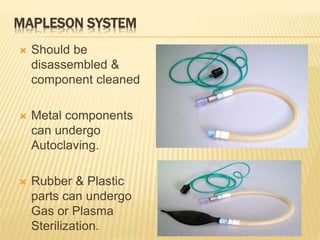
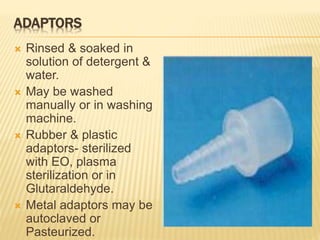
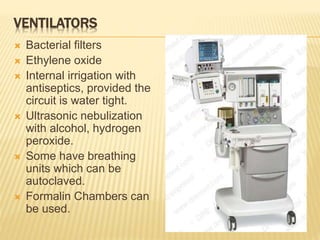
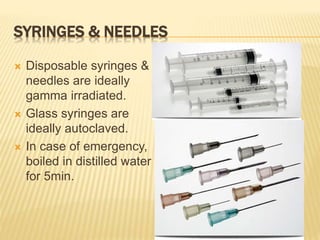
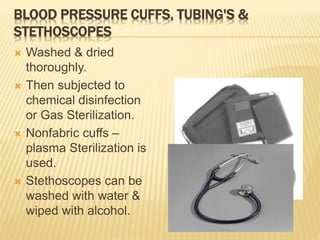
This document defines various terms related to sterilization and disinfection. It discusses different sterilization methods including steam sterilization, dry heat sterilization, chemical sterilization methods using formaldehyde, alcohol, chlorine, iodophors and hydrogen peroxide. It also covers cleaning and disinfection of equipment, factors influencing chemical sterilization, and advantages of chemical sterilization.