In the United States, western Europe, and Japan, butadiene is produced as a byproduct of the steam cracking process used to produce ethylene and other alkenes. When mixed with steam and briefly heated to very high temperatures (often over 900 °C), aliphatic hydrocarbons give up hydrogen to produce a complex mixture of unsaturated hydrocarbons, including butadiene. The quantity of butadiene produced depends on the hydrocarbons used as feed. Light feeds, such as ethane, give primarily ethylene when cracked, but heavier feeds favor the formation of heavier olefins, butadiene, and aromatic hydrocarbons.
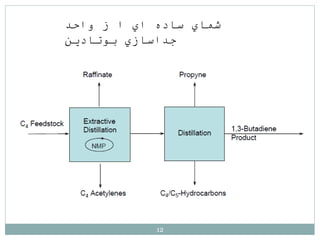
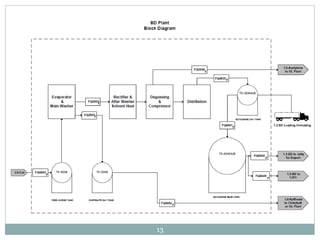
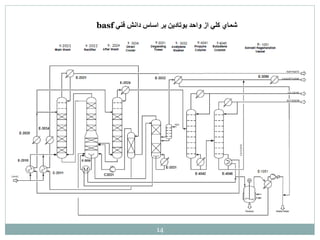
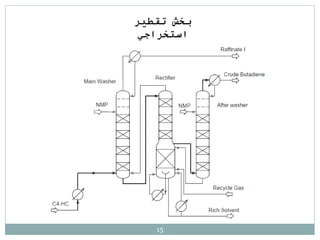
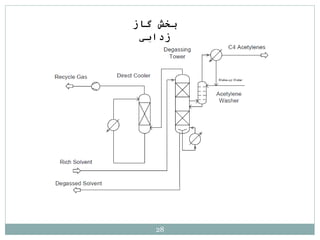
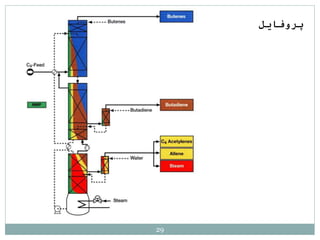
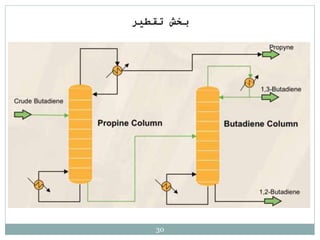
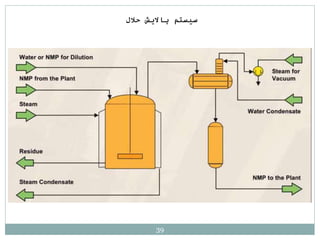
Butadiene is typically isolated from the other four-carbon hydrocarbons produced in steam cracking by extractive distillation using a polar aprotic solvent such as acetonitrile, N-methyl-2-pyrrolidone, furfural, or dimethylformamide, from which it is then stripped by distillation.[14]
From dehydrogenation of n-butane
Butadiene can also be produced by the catalytic dehydrogenation of normal butane (n-butane). The first such post-war commercial plant, producing 65,000 tons per year of butadiene, began operations in 1957 in Houston, Texas.[15] Prior to that, in the 1940s the Rubber Reserve Company, a part of the United States government, constructed several plants in Borger, Texas, Toledo, Ohio, and El Segundo, California, to produce synthetic rubber for the war effort as part of the United States Synthetic Rubber Program.[16] Total capacity was 68 KMTA (Kilo Metric Tons per Annum).
Today, butadiene from n-butane is commercially produced using the Houdry Catadiene process, which was developed during World War II. This entails treating butane over alumina and chromia at high temperatures.[17]
From ethanol
In other parts of the world, including South America, Eastern Europe, China, and India, butadiene is also produced from ethanol. While not competitive with steam cracking for producing large volumes of butadiene, lower capital costs make production from ethanol a viable option for smaller-capacity plants. Two processes were in use.
In the single-step process developed by Sergei Lebedev, ethanol is converted to butadiene, hydrogen, and water at 400–450 °C over any of a variety of metal oxide catalysts