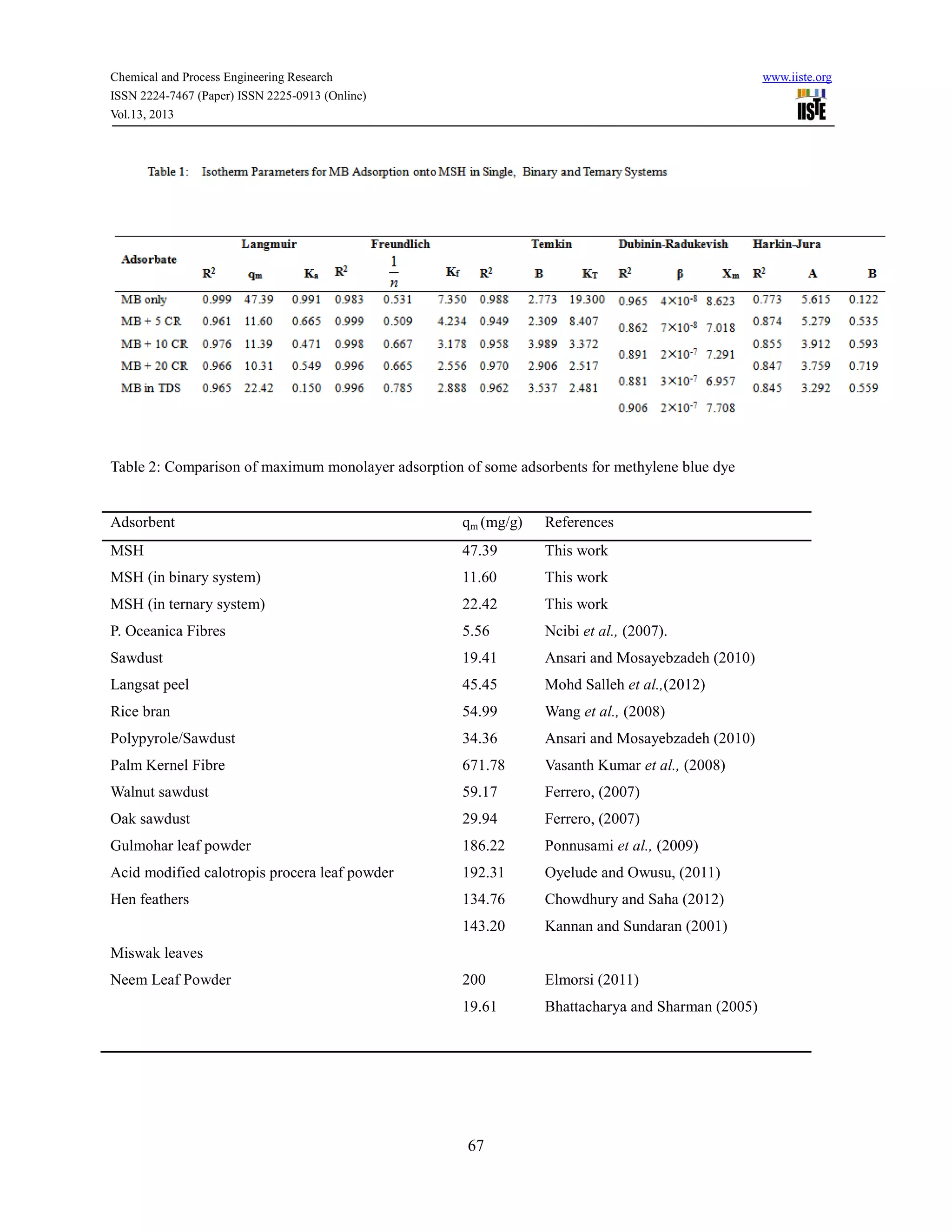
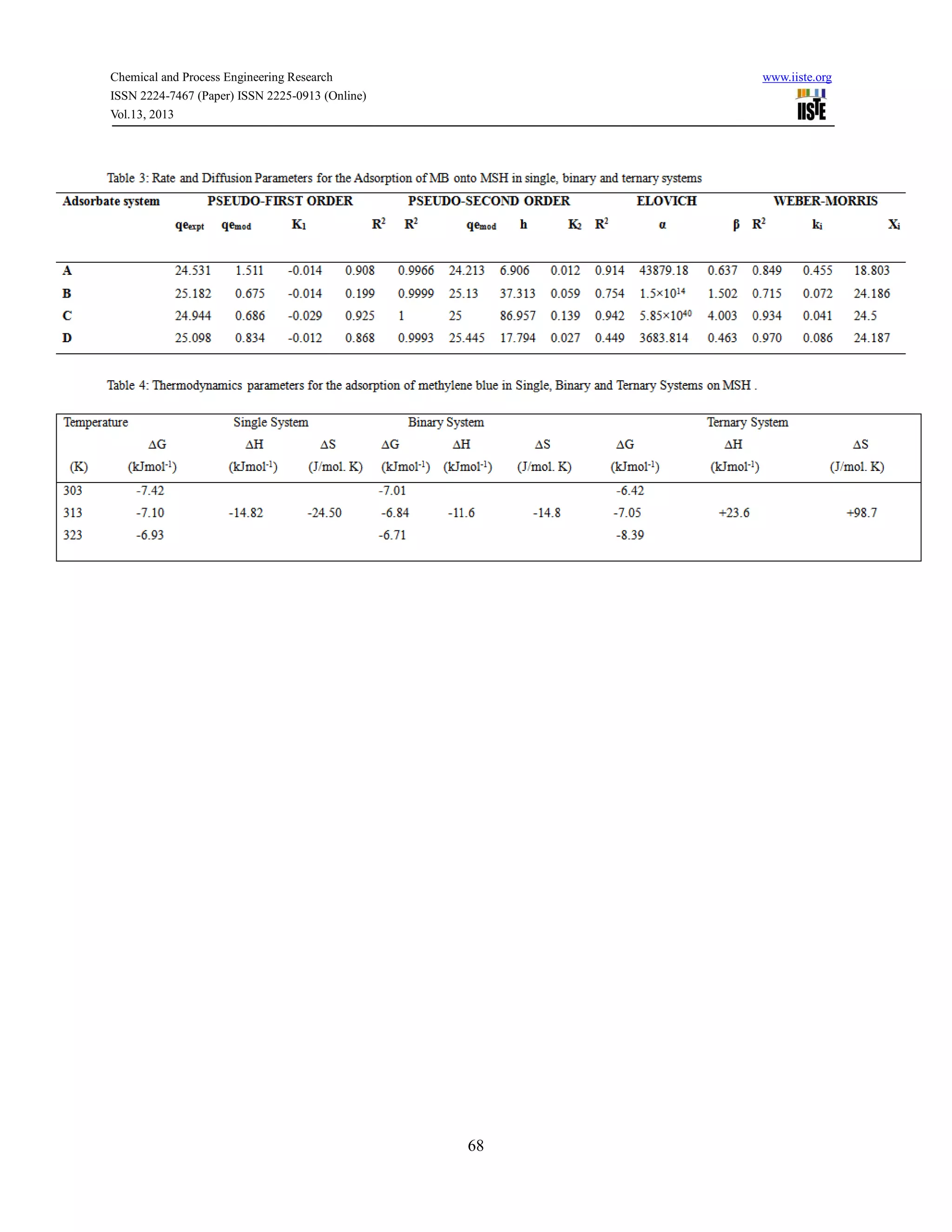
This document discusses a study that investigated the adsorption behavior of the cationic dye methylene blue in single, binary, and ternary solutions using melon husk as an adsorbent. Experiments showed that adsorption equilibrium was reached within 120 minutes for all systems. Kinetic data fit best to a pseudo-second order model. Isotherm data fit best to the Langmuir model for single systems and Freundlich model for binary and ternary systems. Adsorption was found to be thermodynamically feasible and exothermic for single and binary systems but endothermic for ternary systems.












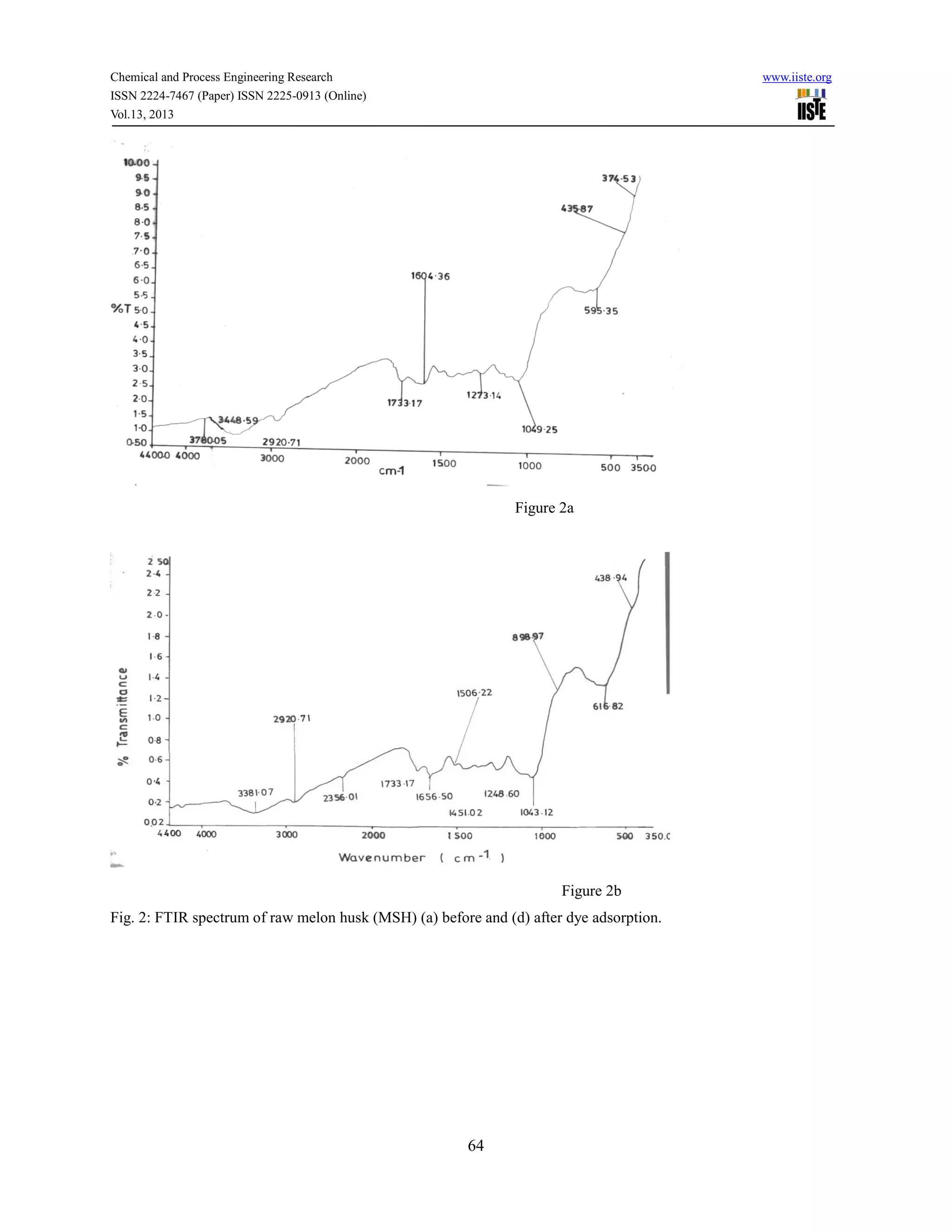
![Chemical and Process Engineering Research www.iiste.org
ISSN 2224-7467 (Paper) ISSN 2225-0913 (Online)
Vol.13, 2013
66
0
5
10
15
20
25
30
0 20 40 60 80 100 120 140
t (min)
qt(mg/g)
A
B
C
D
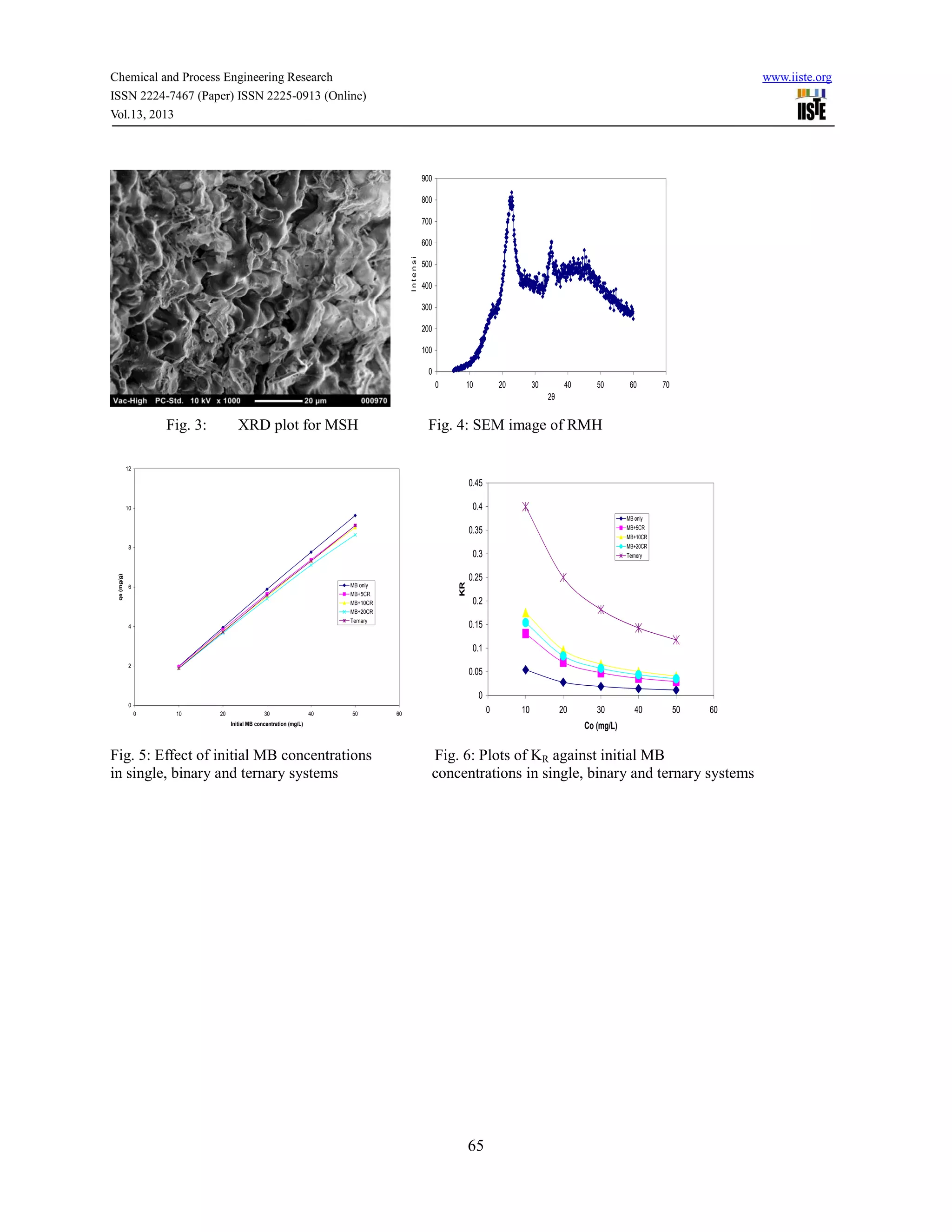
Fig .7: Effect of contact time on MB adsorption onto RMH in SDS and TDS
(A) SDS: ([MB]= 50 mg/L); (B) TDS: ([MB]=50 mg/L; [CR]=10 mg/L and [MO]=10 mg/L); (C) TDS: ([MB]=50
mg/L; [CR]=20 mg/L and [MO]=10 mg/L); (D) TDS: ([MB]=50 mg/L; [CR]=10 mg/L and [MO]=20 mg/L)
-2
-1.5
-1
-0.5
0
0.5
1
1.5
0 20 40 60 80 100 120
qt (min)
log(qe-qt)
A
B
C
D
0
5
10
15
20
25
30
1 1.5 2 2.5 3 3.5 4 4.5 5
ln t
qt(mg/g)
A
B
C
D
(a) (b)
0
1
2
3
4
5
6
0 20 40 60 80 100 120 140
t (min)
t/qt
A
B
C
D
(c)
Fig.8: Plots of Pseudo-first order (a), Elovich (b) and Pseudo-second order (c) for the sorption of MB onto MSH](https://image.slidesharecdn.com/removalofbasicdyefromaqueoussolutionbyadsorptiononmelonhuskin-130901020246-phpapp02/75/Removal-of-basic-dye-from-aqueous-solution-by-adsorption-on-melon-husk-in-16-2048.jpg)