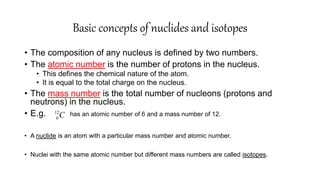
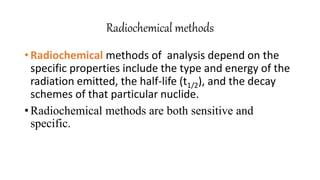
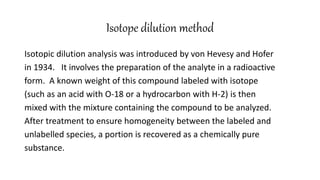
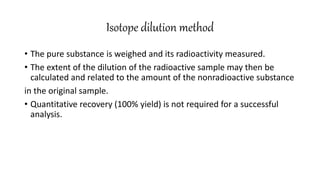
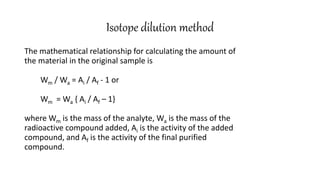
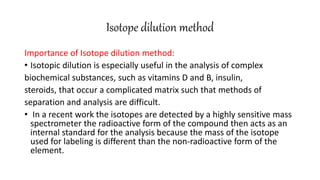
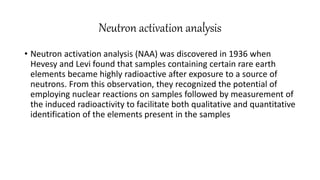
This document discusses radiochemical methods of analysis. It begins by defining nuclides and isotopes. There are three main types of radiochemical methods: radiometric analysis which measures natural radioactivity, isotope dilution which introduces a known radioactive isotope, and activation analysis which induces radioactivity. Isotope dilution involves adding a known amount of a radioactive isotope and measuring the dilution to determine the original amount of the non-radioactive substance. Neutron activation analysis works by inducing radioactivity in samples through neutron irradiation and then detecting and identifying the radioactive elements. Radiochemical methods can be used to analyze various substances like metals in water or nutrients in foods.






![Other methods
• Radio immune assay RIA:
• This method is based upon the completion between radiolabelled antigen
(Ag*) and unlabeled antigens (Ag) for binding to a specific antibody (Ab)
serum.
• Ag* + Ag + Ab [Ag*Ab] + [Ag Ab] + [Ag* + Ag]
. Autoradiography:
• The method is used for the detection of separated compounds on the
surface of thin layer plate or paper can be done by this technique.
• Example:
• 16 different amino acids can be located and separated on thin layer plate
by this method.](https://image.slidesharecdn.com/radiochemicalmethodslec-200107064520/85/Radiochemical-methods-lec-15-320.jpg)