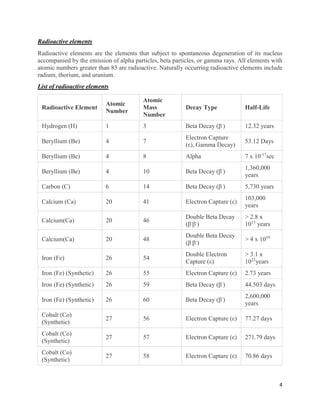
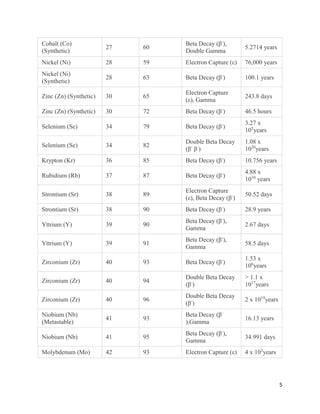
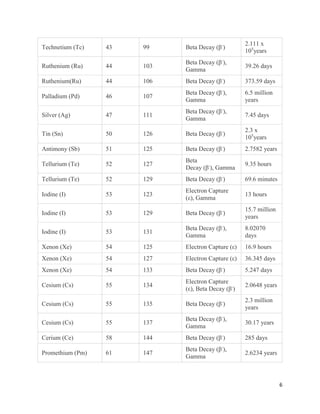
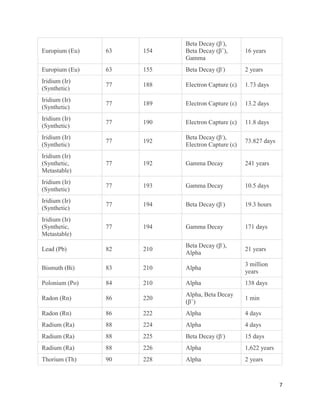
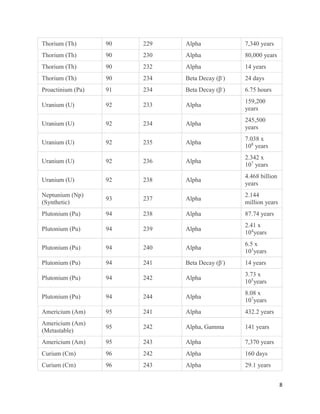
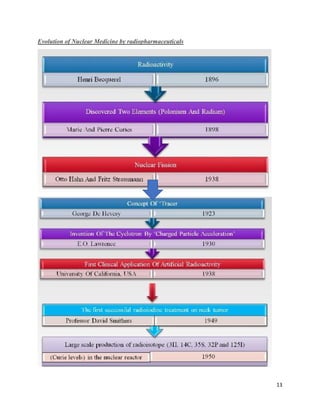
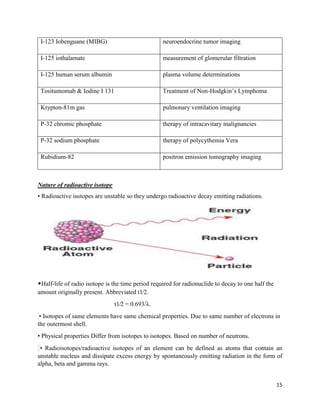
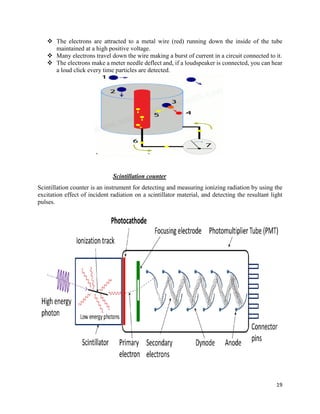
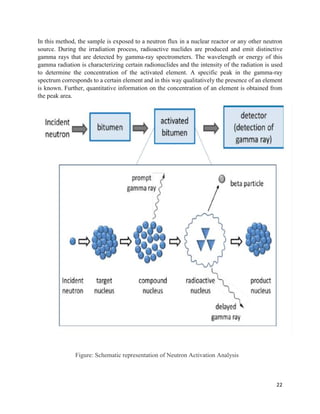
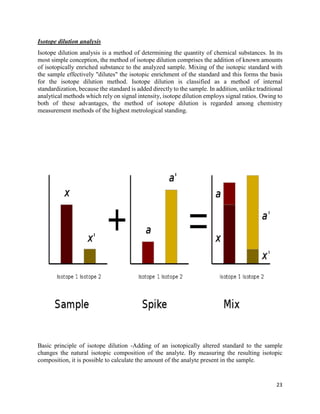
This document provides an overview of radiation and radioactive elements. It discusses the classification of radiation into ionizing (alpha, beta, gamma) and non-ionizing radiation. It lists many naturally occurring radioactive elements and their properties. The document describes different types of radioactive decay including alpha, beta, and gamma decay. It discusses applications of radioisotopes in medicine, industry, and research. Nuclear fusion and fission are also summarized. The document provides information on radioactivity, radiopharmaceuticals, and detection of radiation.