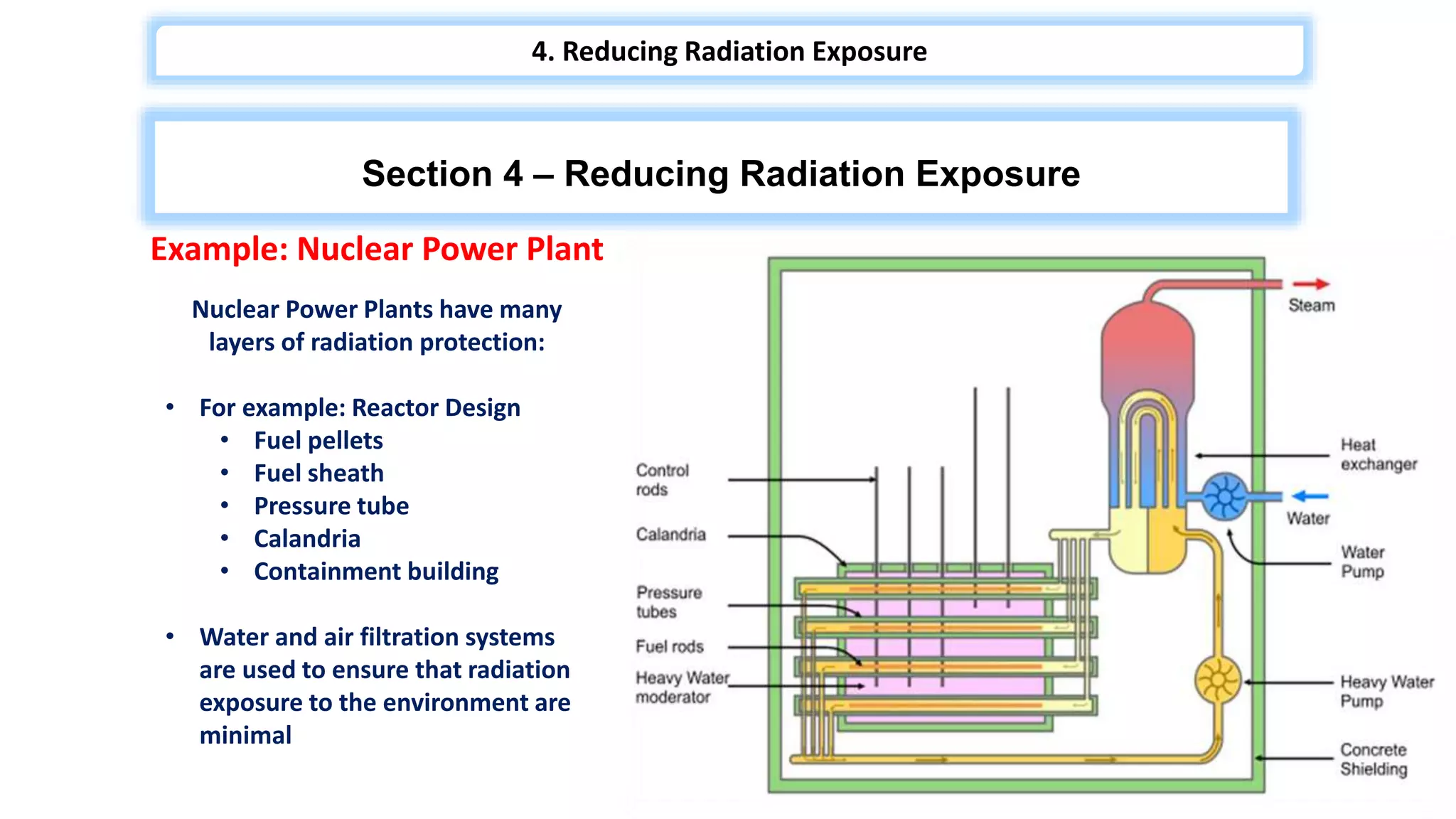
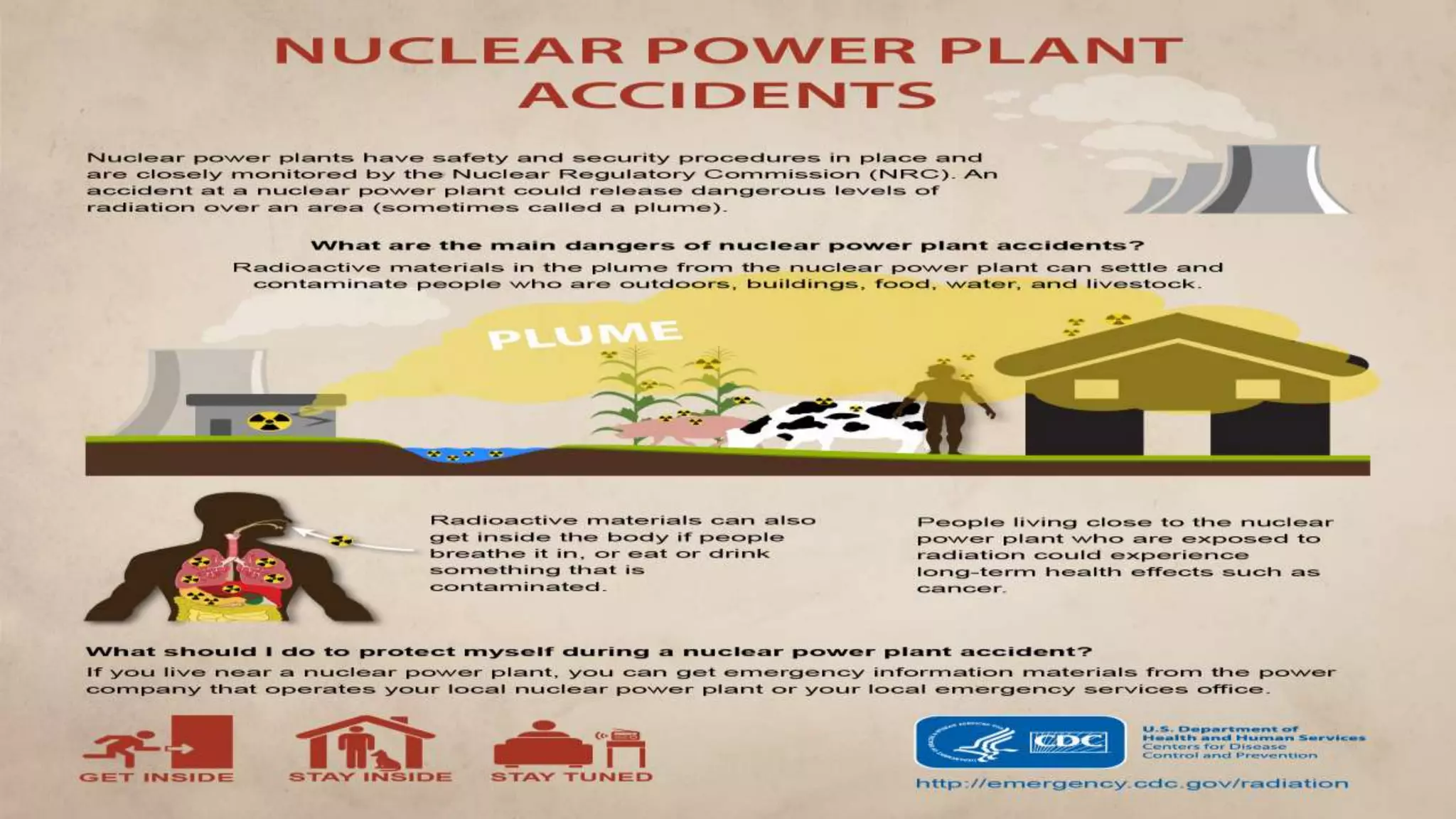
The document discusses radiation hazards and shielding in nuclear power plants, detailing types of radiation, biological effects, regulations, and methods for reducing radiation exposure based on the ALARA principle. It also highlights the importance of safety regulations, worker training, and the response to nuclear accidents, specifically referencing the Fukushima Daiichi disaster. The need for positive public perception regarding nuclear energy is emphasized as a crucial aspect of harnessing clean energy.
![RADIATION HAZARDS & SHIELDING IN NUCLEAR POWER PLANT
Presented By :
ABDUL AWAL
H.T.-14H11A0301
CLASS-B.TECH[IV-I]
BRANCH-MECHANICAL
SECTION-A
JAWAHARLAL NEHRU TECHNOLOGICAL UNIVERSITY HYDERABAD](https://image.slidesharecdn.com/radiationhazardsshieldinginnuclearpowerplant-190614081254/75/Radiation-hazards-shielding-in-nuclear-power-plant-1-2048.jpg)