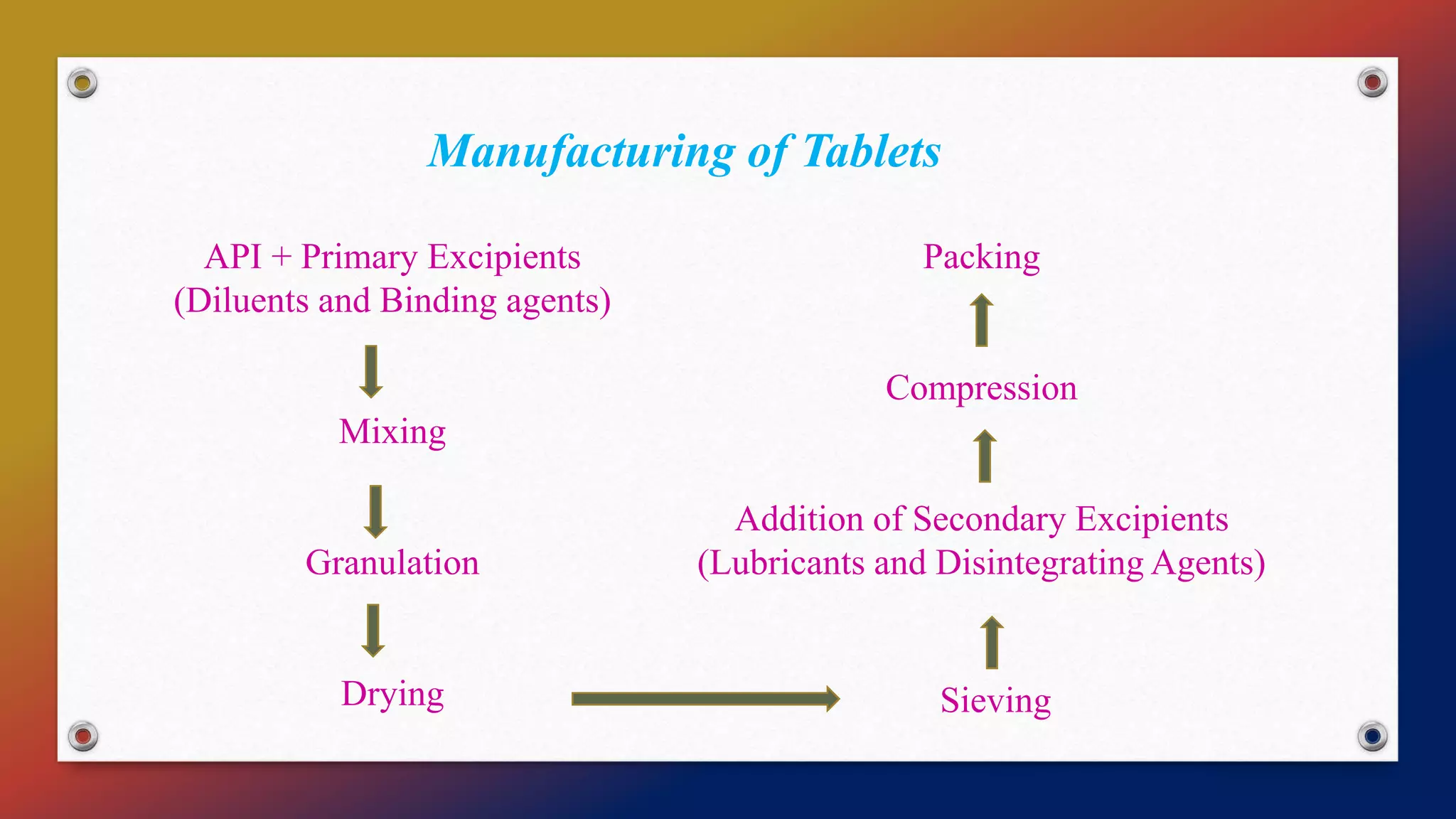
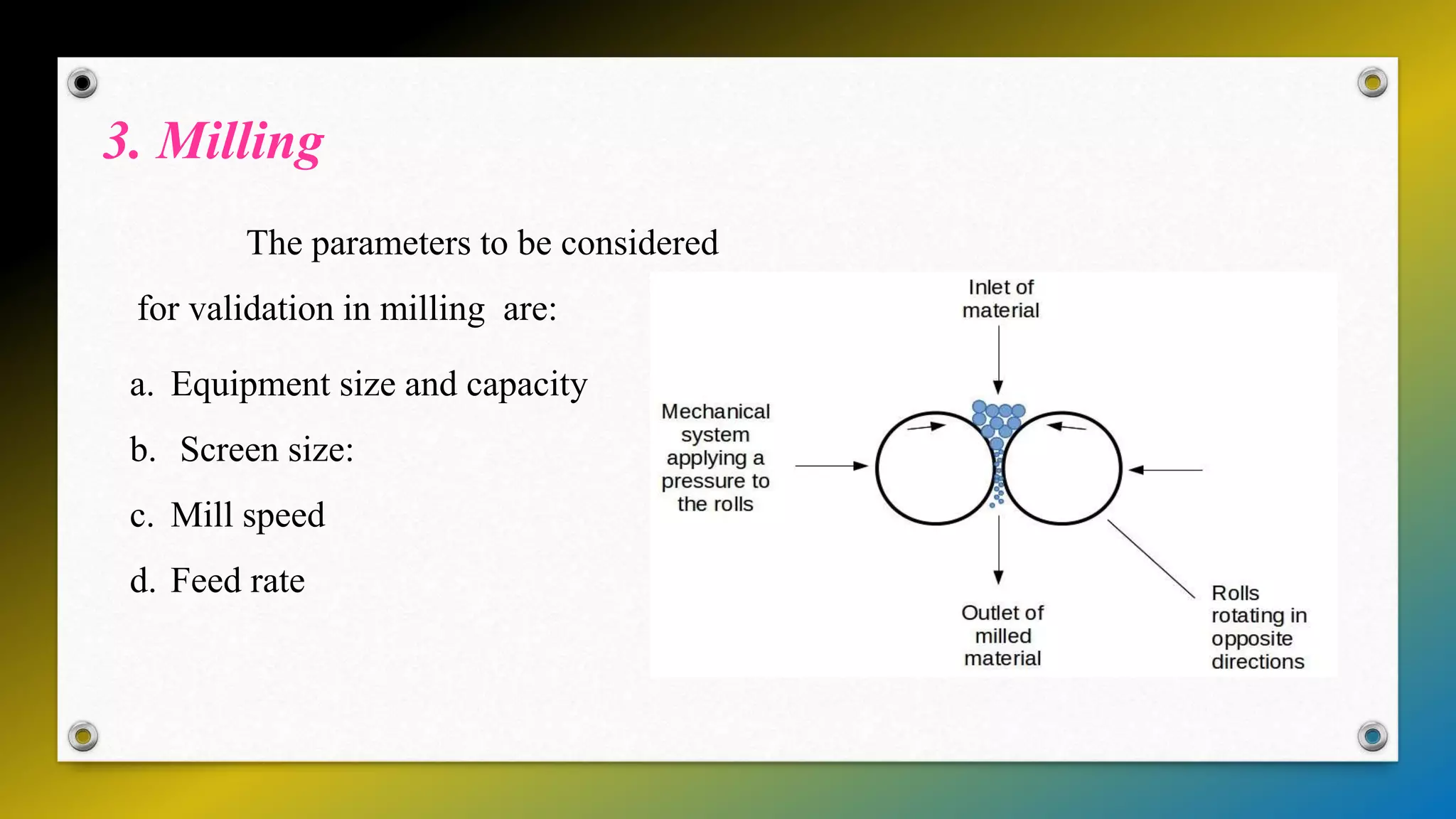
This document discusses process validation for tablets, capsules, and parenterals. It defines validation and process validation, and describes the key steps in manufacturing each dosage form. For tablets, these include mixing, granulation, milling, drying, compression, and coating. Parameters to validate at each step are identified. In-process and finished product tests are also outlined. Validation of capsules involves composition and encapsulation processes. Parenteral validation focuses on sterilization, manufacturing, and packaging. References on pharmaceutical process validation and specific dosage forms are provided.