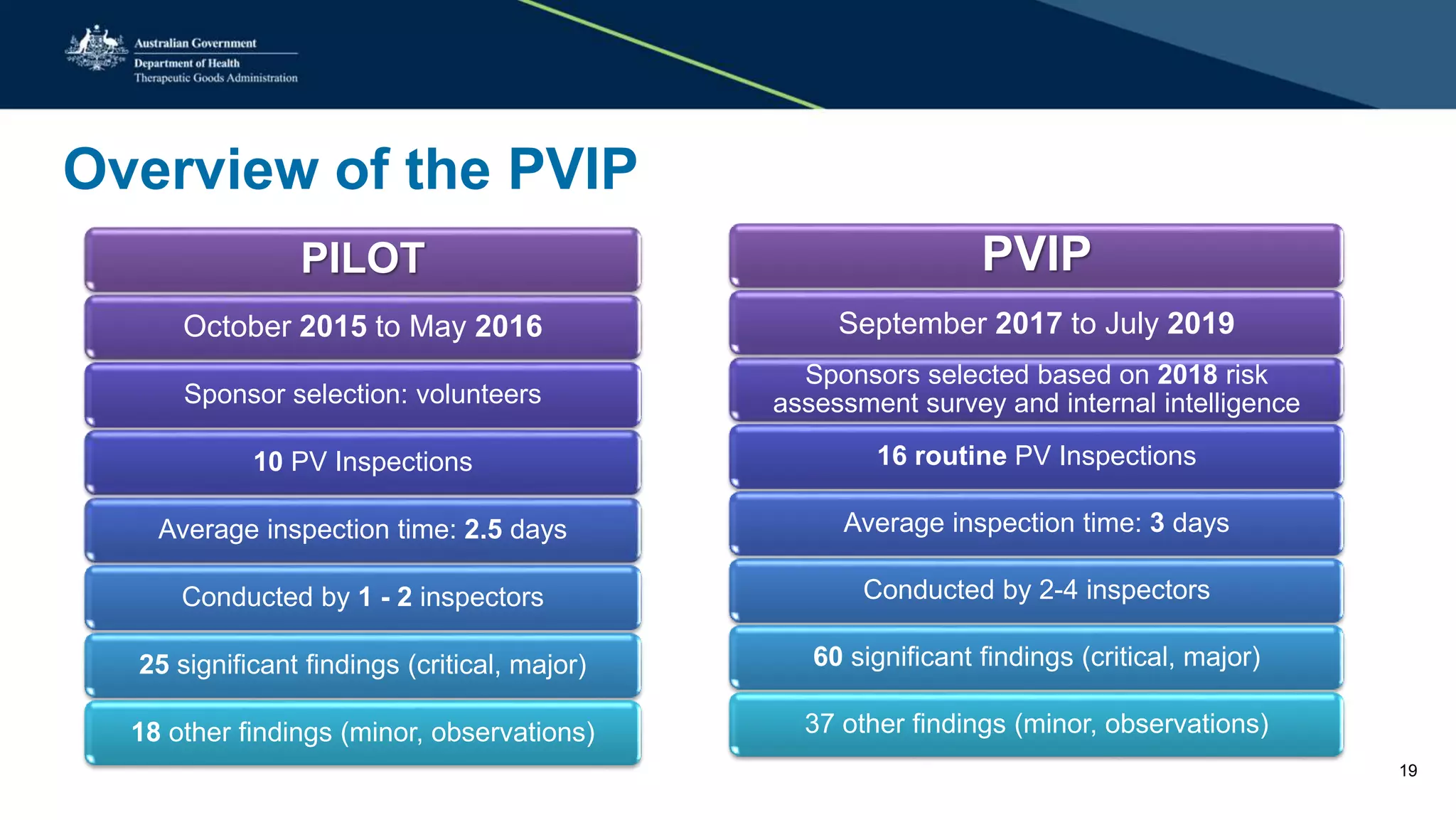
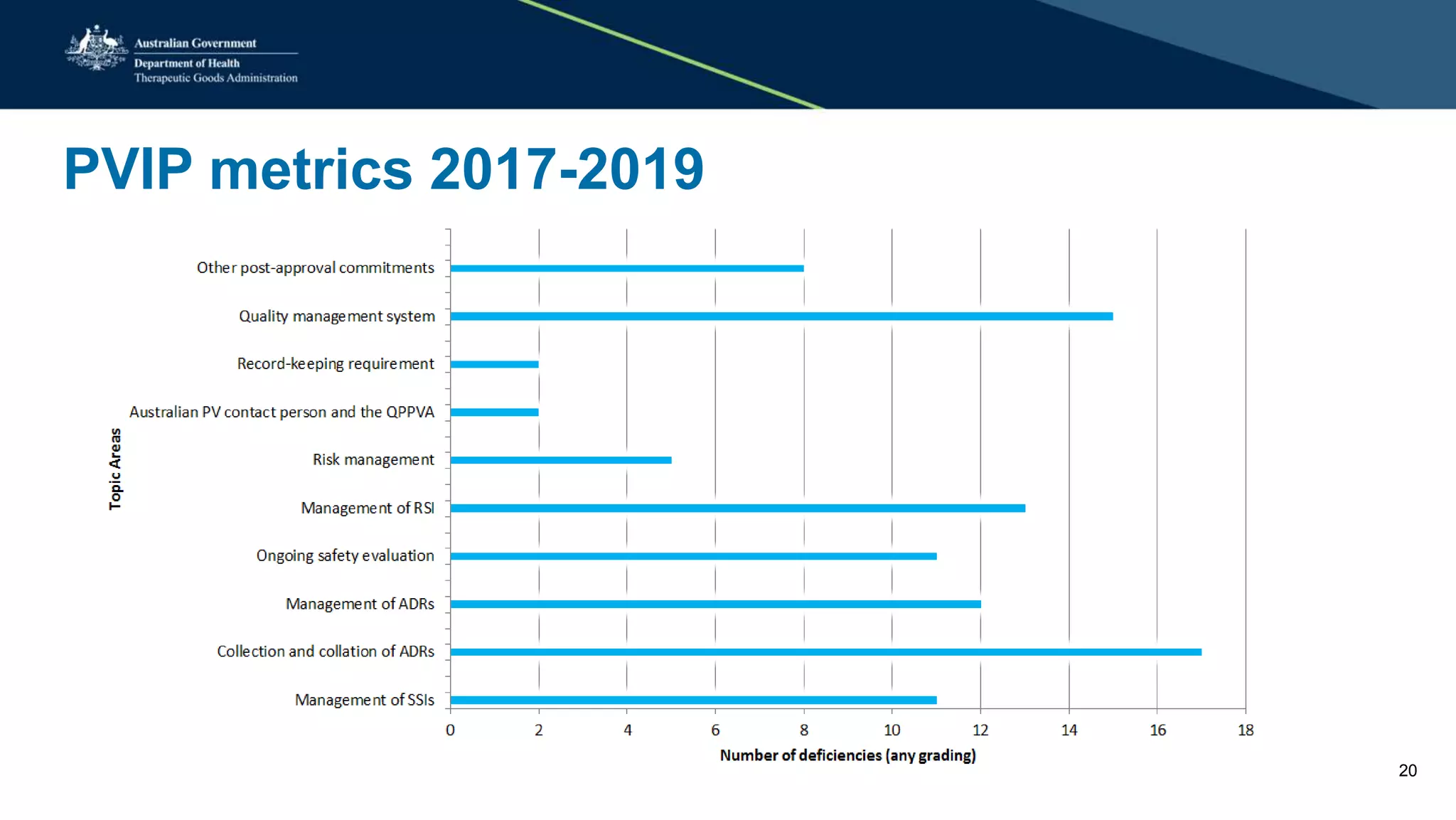
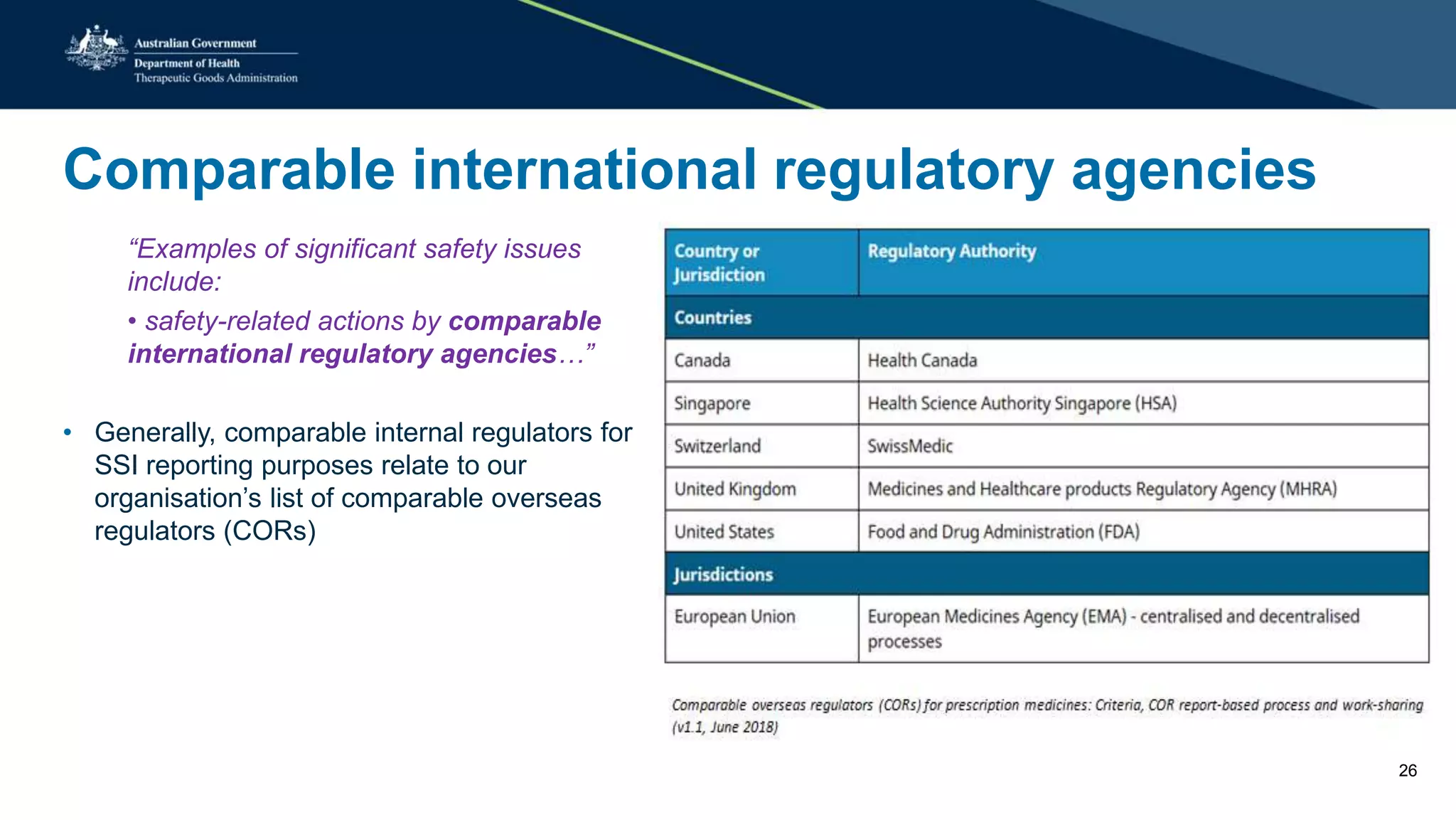
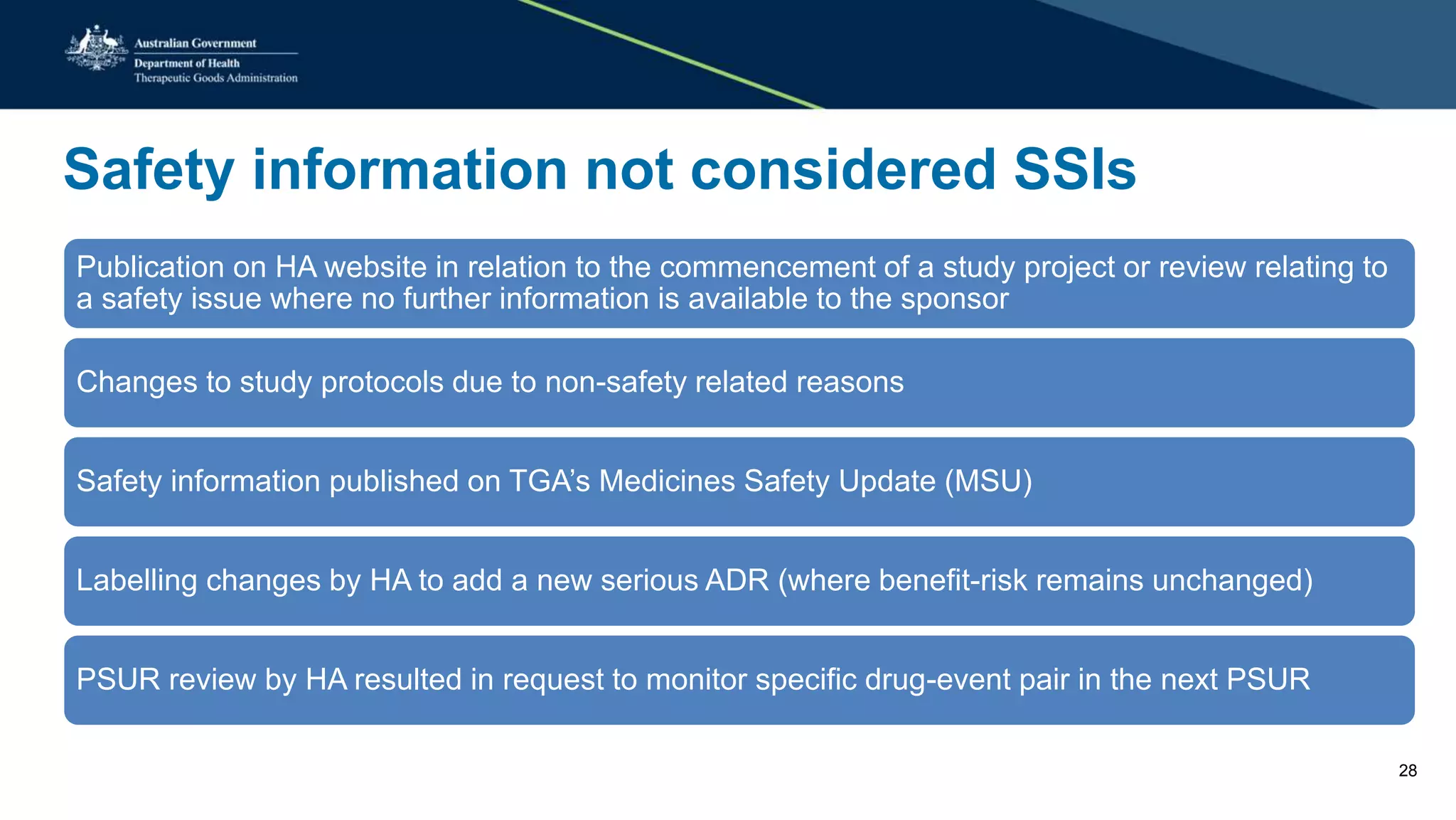
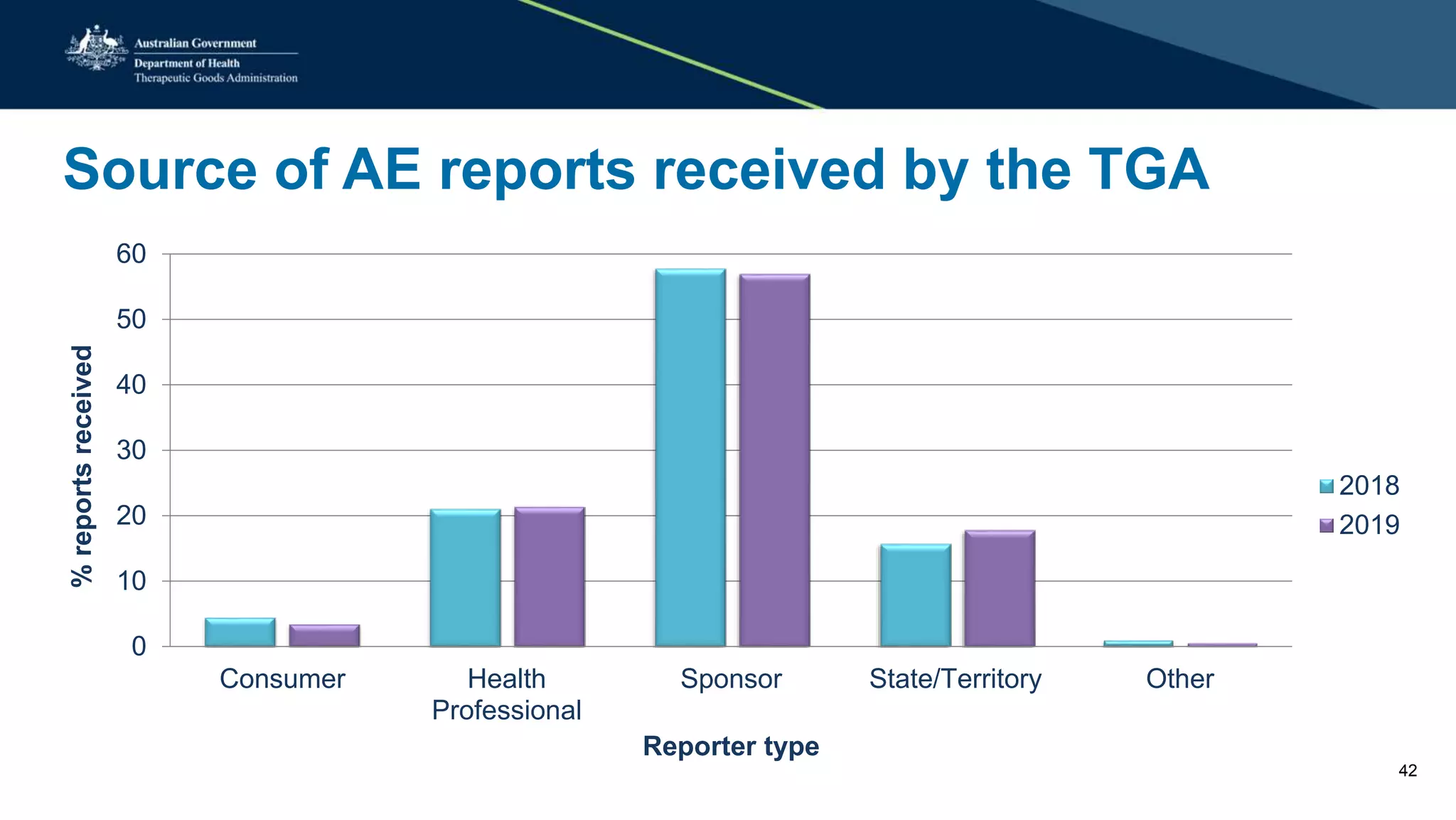
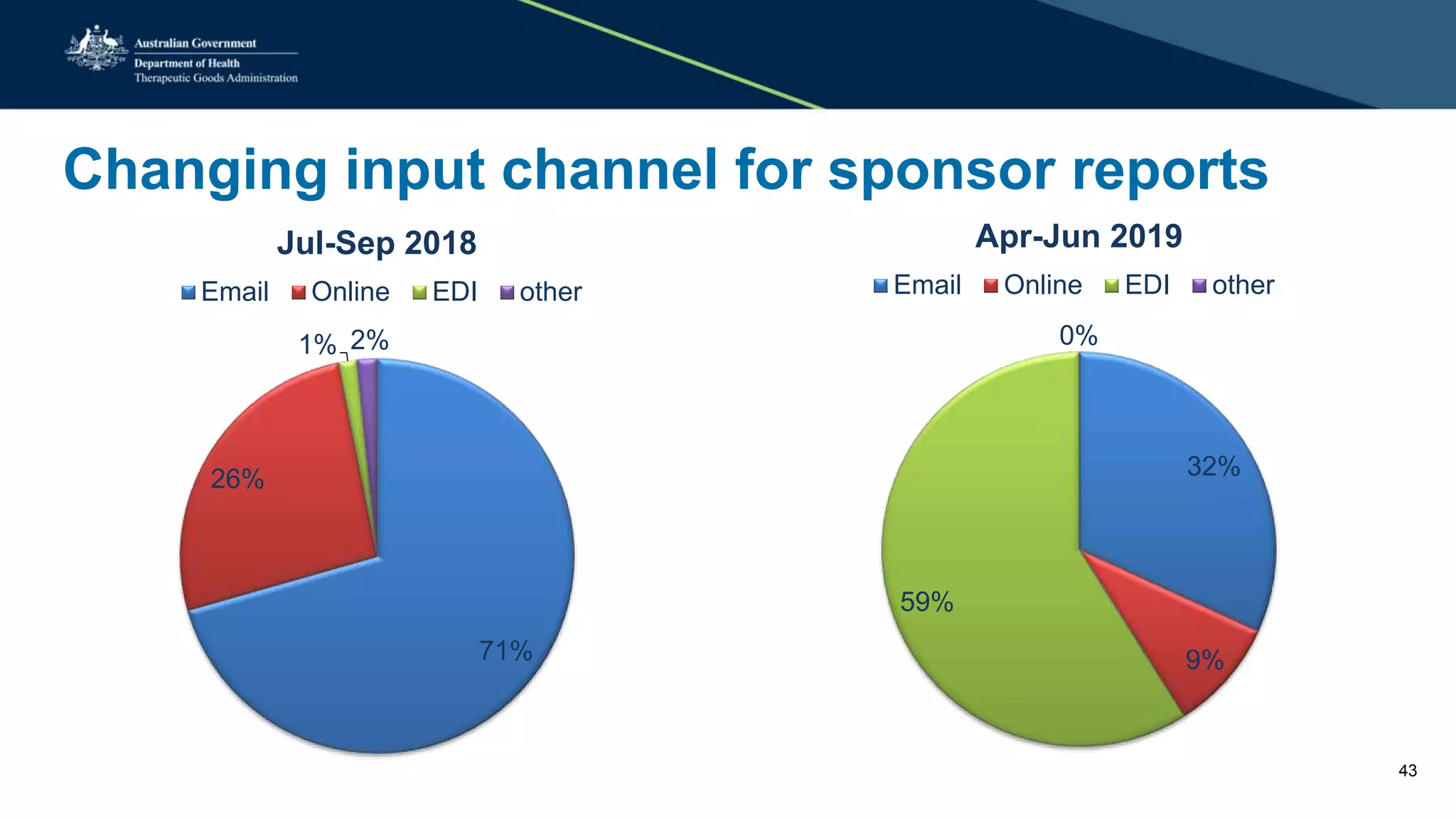
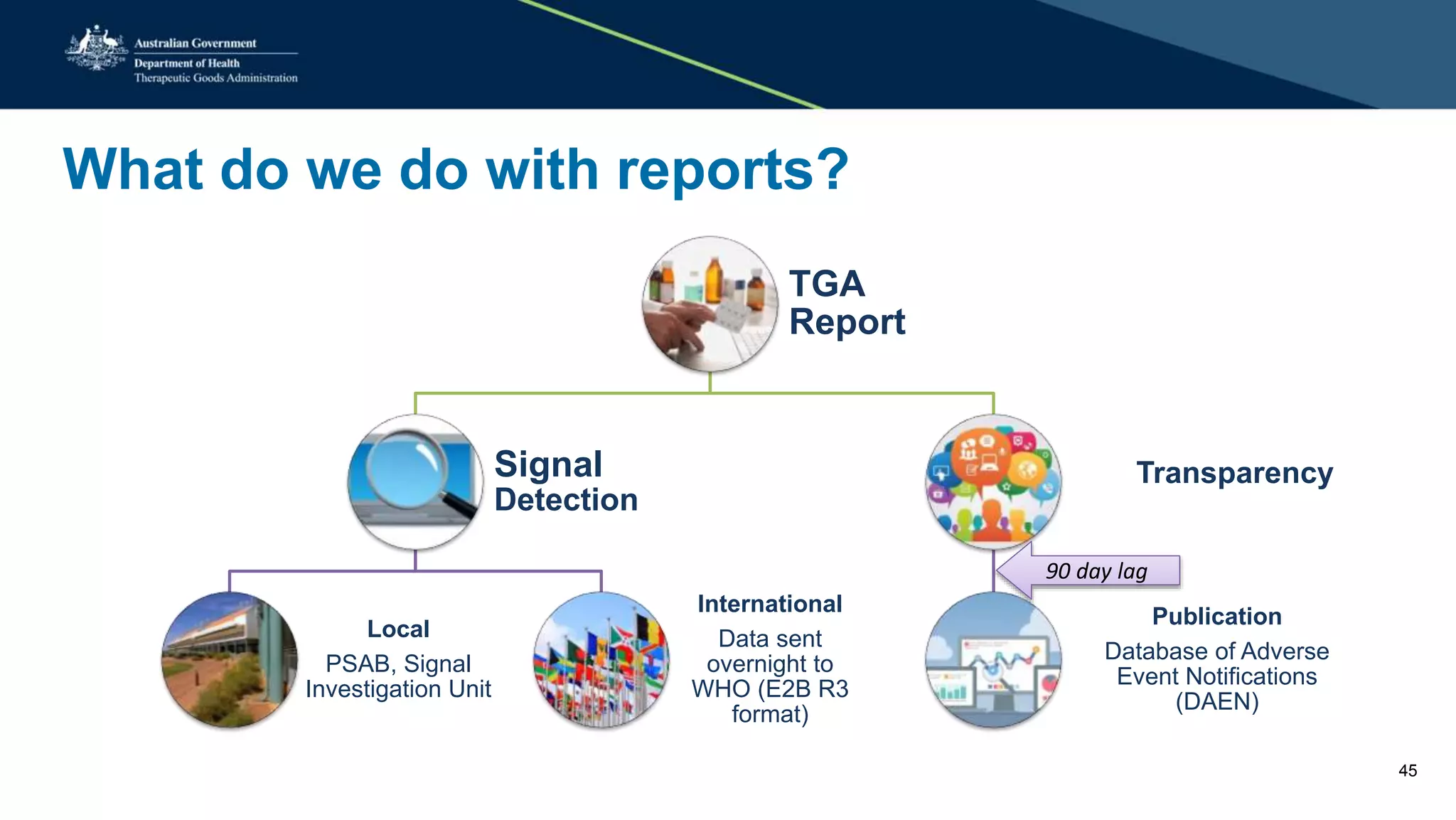
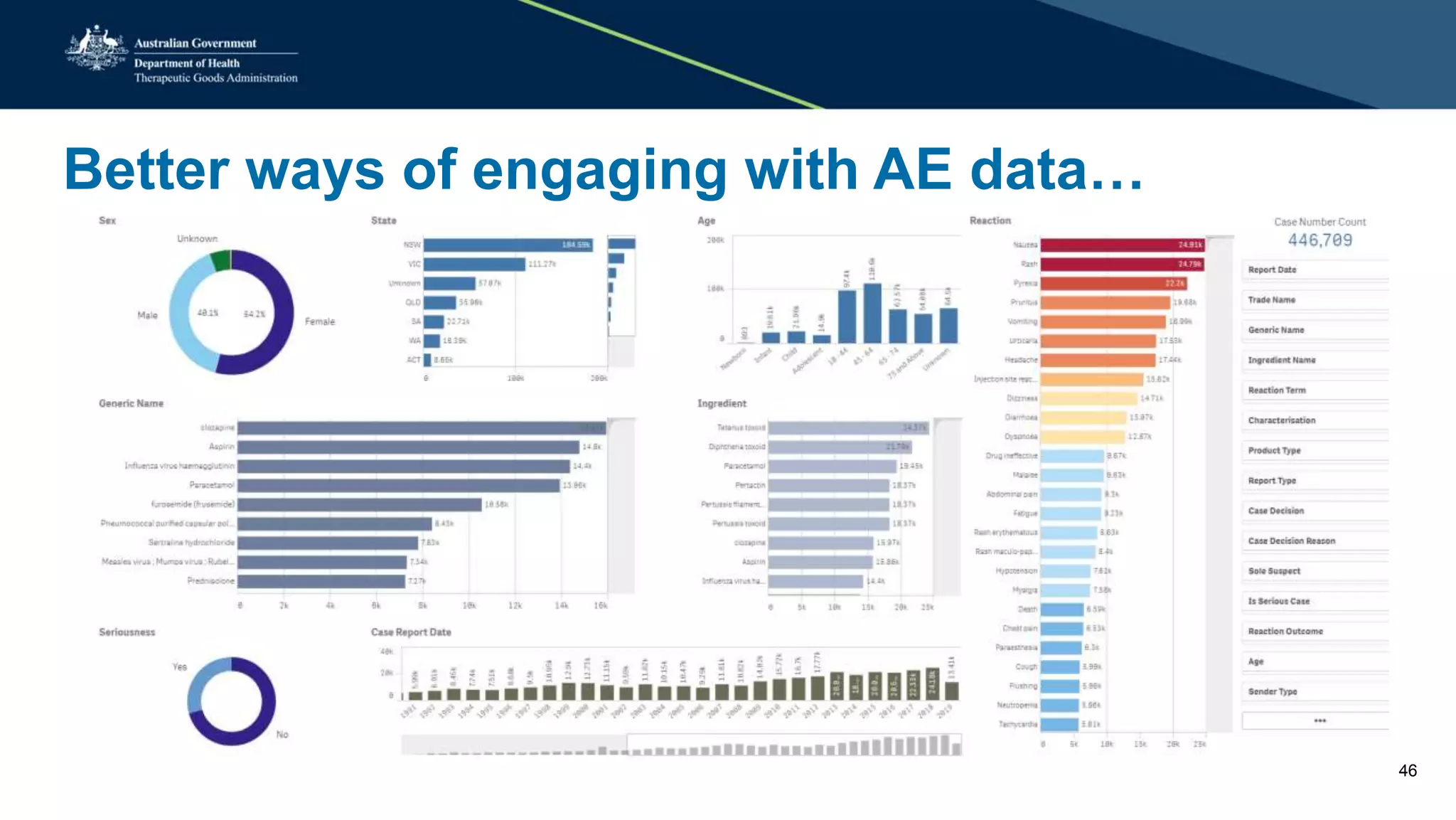
The document provides updates from the Australian pharmacovigilance and special access branch, focusing on the use of new data sources for pharmacovigilance, including the development of a comprehensive post-market monitoring scheme. It discusses signal detection and validation studies utilizing various health data sets, emphasizing the importance of collaboration and expertise in pharmacovigilance activities. Additionally, it outlines findings from inspections and the management of significant safety issues within the regulatory framework.