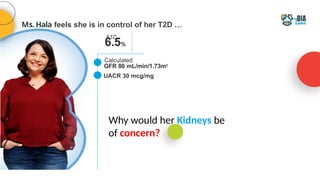
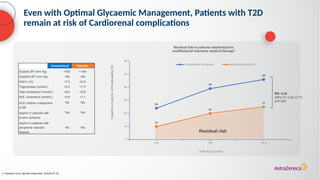
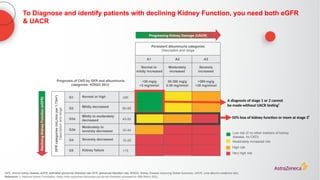
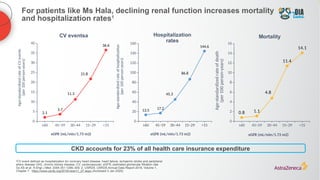
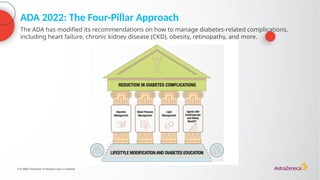
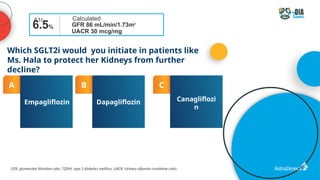
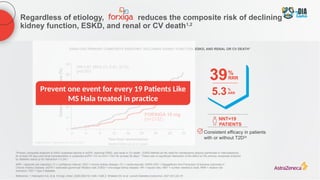
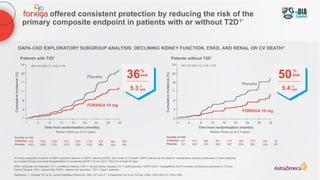
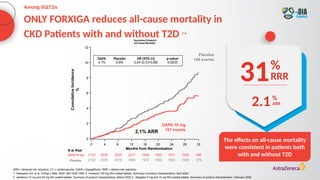
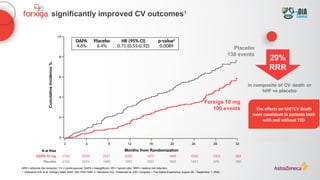
The document discusses a case presentation of a 45-year-old woman with type 2 diabetes (T2D) and risk factors for chronic kidney disease (CKD). It emphasizes the importance of monitoring kidney function in diabetic patients and the need for appropriate prescribing practices to mitigate risks of CKD progression. The guidelines suggest individualized treatment plans considering cardiovascular and renal outcomes for T2D management.







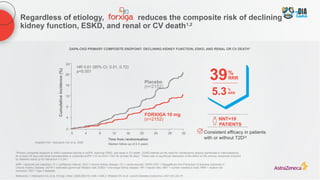
![Diabetes is a strong risk factor for CKD and increases the risk of
progression to ESKD1
a
CKD was defined as eGFR of 15–59 mL/min/1.73 m2
(stages 3–4); b
HRs were adjusted for age, sex, race, smoking, history of CVD, serum total cholesterol concentration, body mass index, and albuminuria (log urine albumin:creatinine ratio, log urine protein:creatinine ratio, or categorical dipstick proteinuria [negative, trace, 1+, ≥2+]);c
Blue and purple circles denote
P<0.05 as compared with the reference (diamond); d
eGFR 50 mL/min/1.73 m2
used as the reference point (diamond) in diabetes and no diabetes groups
CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; ESKD, end stage kidney disease; T2D, Type 2 diabetes
1. Murphy D, et al. Ann Intern Med. 2016;165:473–481; 2. Fox CS, et al. Lancet 2012;380:1662–1673
No diabetes Diabetes
0
10
20
30
40
50
5.3
19.1
Proportion
of
patients
(%)
Prevalence of CKD is more than three
times higher in patients with diabetes1
Prevalence of CKD stages 3–41,a
2011–2012
Risk of ESKD according to baseline eGFR
in individuals with and without diabetes2,b–d
Diabetes, 95% CI
No diabetes, 95% CI
Adjusted
hazard
ratio
eGFR (mL/min/1.73 m2
)
15 30 45 60
0
0.5
64
32
16
8
4
2
1
Risk of ESKD is higher in patients with
diabetes2](https://image.slidesharecdn.com/astrazencapresentationdiatunemshalat2dwdecliningkidneyfunction1-250202101652-511188f8/85/Presentation-T2D_W_Declining_Kidney_Function-1-pptx-11-320.jpg)






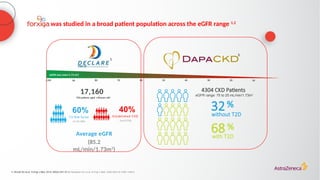
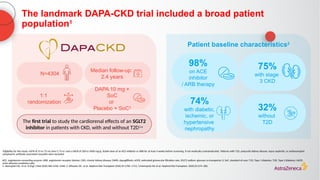
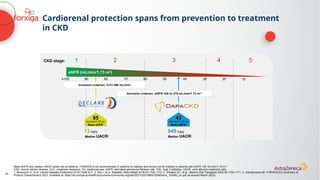
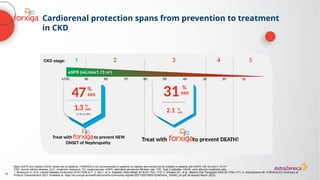
![The cardiorenal benefits of FORXIGA were initially observed in patients
with early renal disease and T2D in DECLARE TIMI 58
‑
a
Baseline UACR data were missing from 316 (2%) patients; b
Nominally significant, prespecified exploratory renal composite outcome of a sustained decrease of ≥40% in eGFR to
<60 mL/min/1.73 m2
, new ESKD, or death from kidney causes; c
hHF alone was a separate, nominally significant exploratory endpoint in DECLARE. The primary endpoint composite of CV death/hHF (17% RRR [0.9% ARR]) was driven by hHF
ARR, absolute risk reduction; CI, confidence interval; CV, cardiovascular; eGFR, estimated glomerular filtration rate; ESKD, end-stage kidney disease; hHF, hospitalization for heart failure; HR, hazard ratio; RRR, relative risk reduction; T2D, Type 2
diabetes; UACR, urine albumin:creatinine ratio
1. Wiviott SD, et al. N Engl J Med 2019;380:347–357; 2. Raz I, et al. Diabetes Obes Metab 2018;20:1102–1110
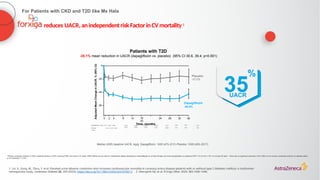
N=17,1601
Renal-specific
composite outcome1,b
(1.5% vs 2.8%)
1.3%
ARR
47RRR
%
<30
(68% of
patients)
30–≤300
(23% of
patients)
>300
(7% of
patients)
UACR2,a
(mg/g)](https://image.slidesharecdn.com/astrazencapresentationdiatunemshalat2dwdecliningkidneyfunction1-250202101652-511188f8/85/Presentation-T2D_W_Declining_Kidney_Function-1-pptx-24-320.jpg)

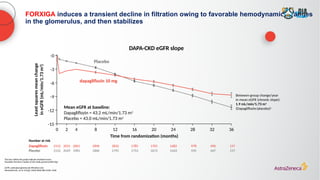
![Select AEs
DECLARE-TIMI 58
(CVOT in T2D)1 DAPA-HF (HFrEF)2
DAPA-CKD3
DELIVER4
Dapagliflozin
10mg
(n=8574)
Placebo
(n=8569)
Dapagliflozin
10mg
(n=2368)
Placebo
(n=2368)
Dapagliflozin 10
mg
(n=2149)
Placebo
(n=2149)
Dapagliflozin
10mg
(N=3131)
Placebo (n=3127)
Diabetic
ketoacidosis
0.3% 0.1% 0.1% 0.0% 0.0% <0.1% 0.1% 0.0%
Severe
hypoglycemiaa 0.7% 1.0% 0.2% 0.2% 0.7% 1.3% 0.2% 0.2%
Volume depletion 2.5% 2.4% 7.5% 6.8% 5.9% 4.2% 1.3% 1.0%
Amputation 1.4% 1.3% 0.5% 0.5% 1.6% 1.8% 0.6% 0.8%
Fracture 5.3% 5.1% 2.1% 2.1% 4.0% 3.2% TBD TBD
Hyperkalemia No hyperkalemia (not listed in SmPC)
No increase in either mild or
moderate/severe hypokalemia observed
TBD TBD
Renal AE 1.5%b
2.0%b
6.5% 7.2% 7.2% 8.7% 2.3% 2.5%
Serious UTI 0.9% 1.3% 0.5% 0.7% 0.9% 0.7% TBD TBD
FORXIGA has demonstrated a consistent safety profile in >32,000 patients across
DECLARE-TIMI 58, DAPA-HF, DAPA-CKD, and DELIVER1–4
a
Severe hypoglycemia was defined in DAPA-CKD and DELIVER as hypoglycemia with the following criteria, confirmed by the investigator: symptoms of severe impairment in consciousness or behavior, need for external assistance, use of an intervention to treat hypoglycemia, and
prompt recovery of acute symptoms following the intervention.2,4,5
Severe hypoglycemia was defined in DECLARE and DAPA-HF as hypoglycemia requiring the assistance of another person to actively administer carbohydrates or glucagon or to take other corrective action. 1,2
All cases of
major hypoglycemia in DAPA-CKD and DAPA-HF occurred in patients with diabetes at baseline 2,3
; b
Acute kidney injury1
AE, adverse event; CVOT, cardiovascular outcomes trial; HFrEF, heart failure with reduced ejection fraction; SmPC, Summary of Product Characteristics; T2D, Type 2 diabetes; TBD, to be determined; UTI, urinary tract infection
1. Wiviott SD, et al. N Engl J Med 2019;380:347–357; 2. McMurray J, et al. N Engl J Med 2019;381:1995–2008; 3. Heerspink HJL, et al. N Engl J Med 2020;383:1436–1446; 4. Solomon S, et al. N Engl J Med 2022;387:1089–1098;
5. AstraZeneca UK Limited. FORXIGA Summary of Product Characteristics [YEAR]. Available at: [Placeholder for link subject to final approval by the European Medical Agency]; 6. AstraZeneca Pharmaceuticals LP. Data on File
It is estimated that >23 million patients were treated with FORXIGA and XIGDUO across indications throughout 20236
Prespecified selected safety outcomes across dapagliflozin clinical trials](https://image.slidesharecdn.com/astrazencapresentationdiatunemshalat2dwdecliningkidneyfunction1-250202101652-511188f8/85/Presentation-T2D_W_Declining_Kidney_Function-1-pptx-39-320.jpg)






![Presentation T2D_W_Declining_Kidney_Function[1].pptx](https://image.slidesharecdn.com/astrazencapresentationdiatunemshalat2dwdecliningkidneyfunction1-250202101652-511188f8/85/Presentation-T2D_W_Declining_Kidney_Function-1-pptx-46-320.jpg)