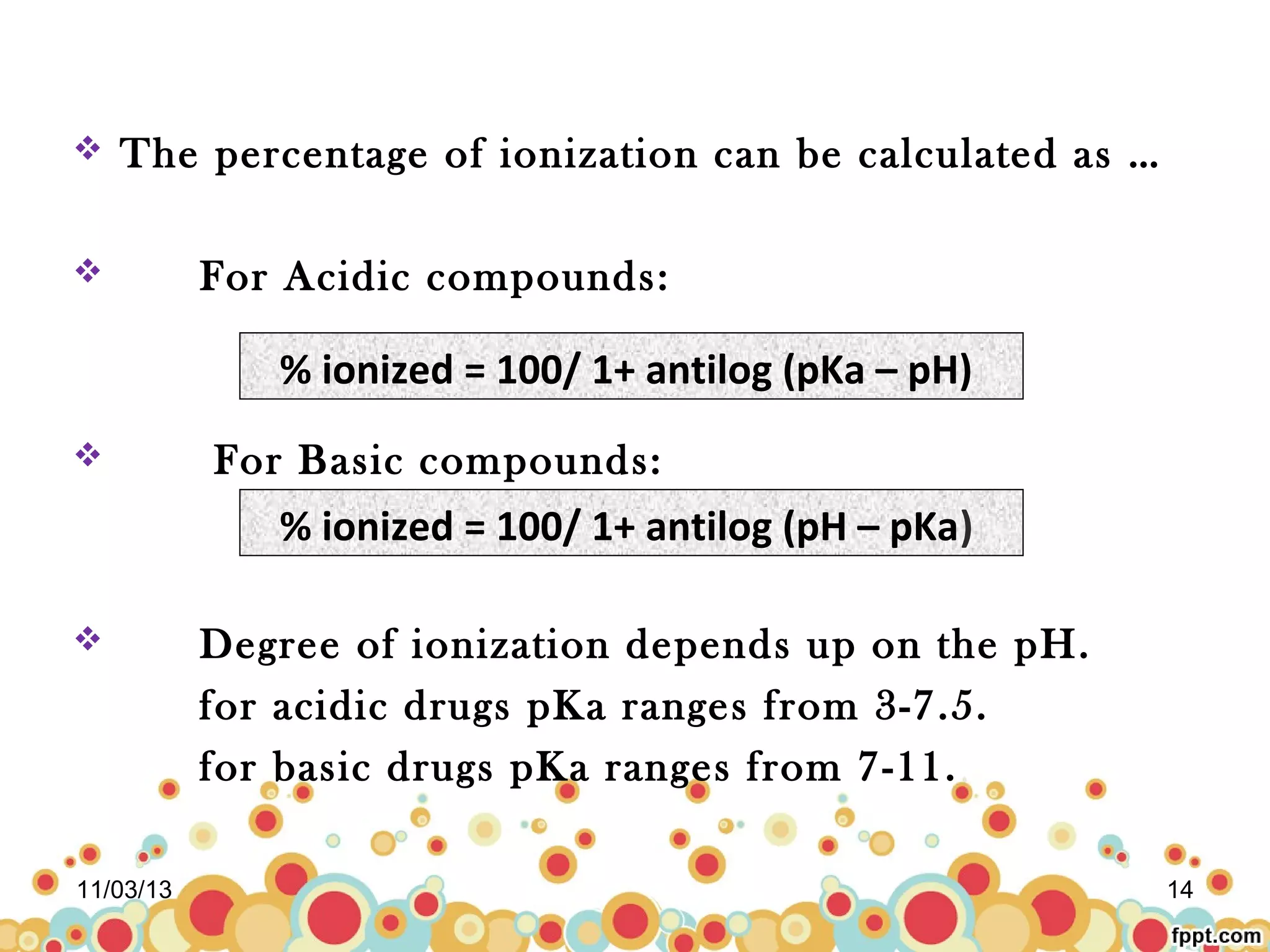
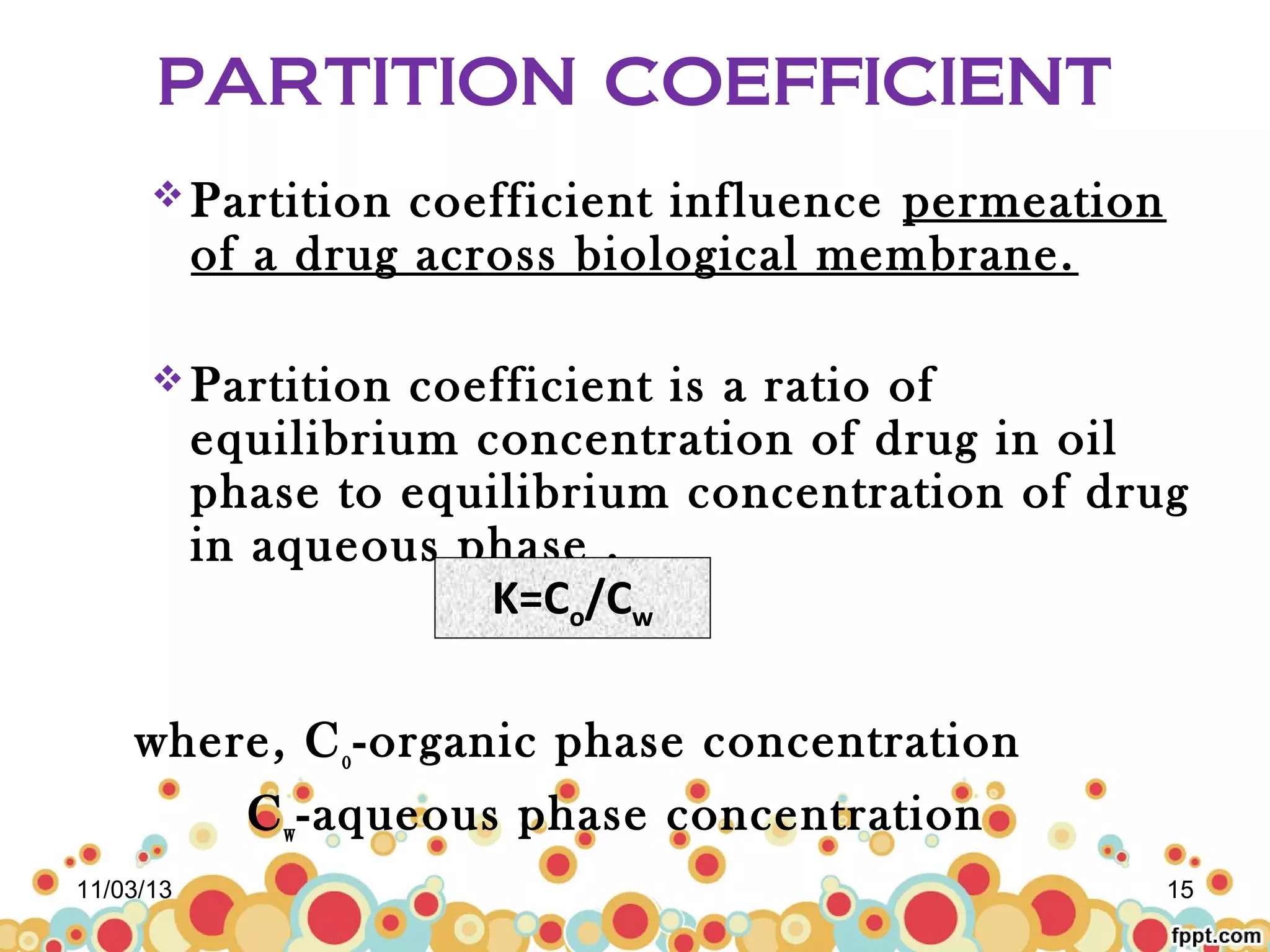
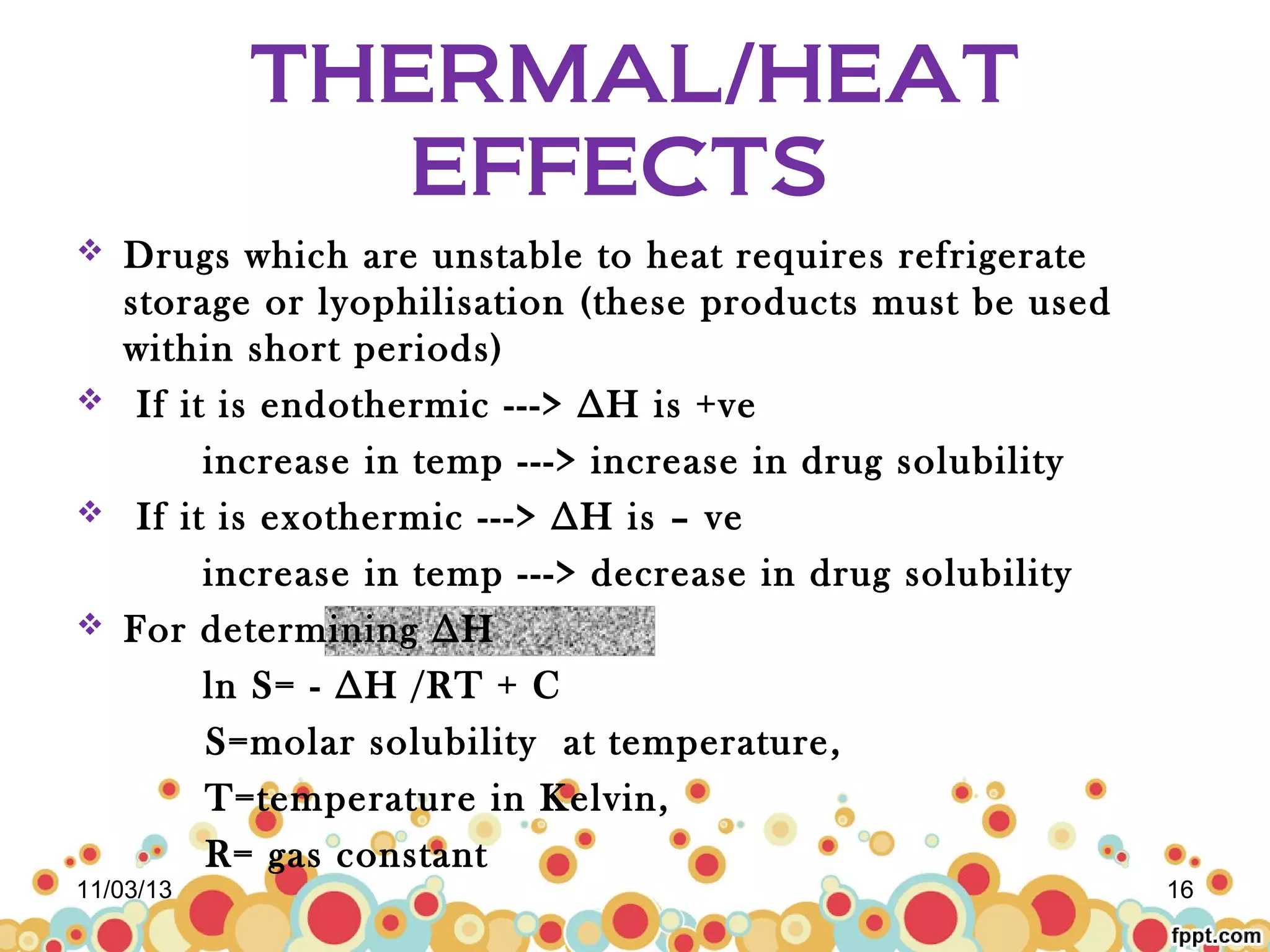
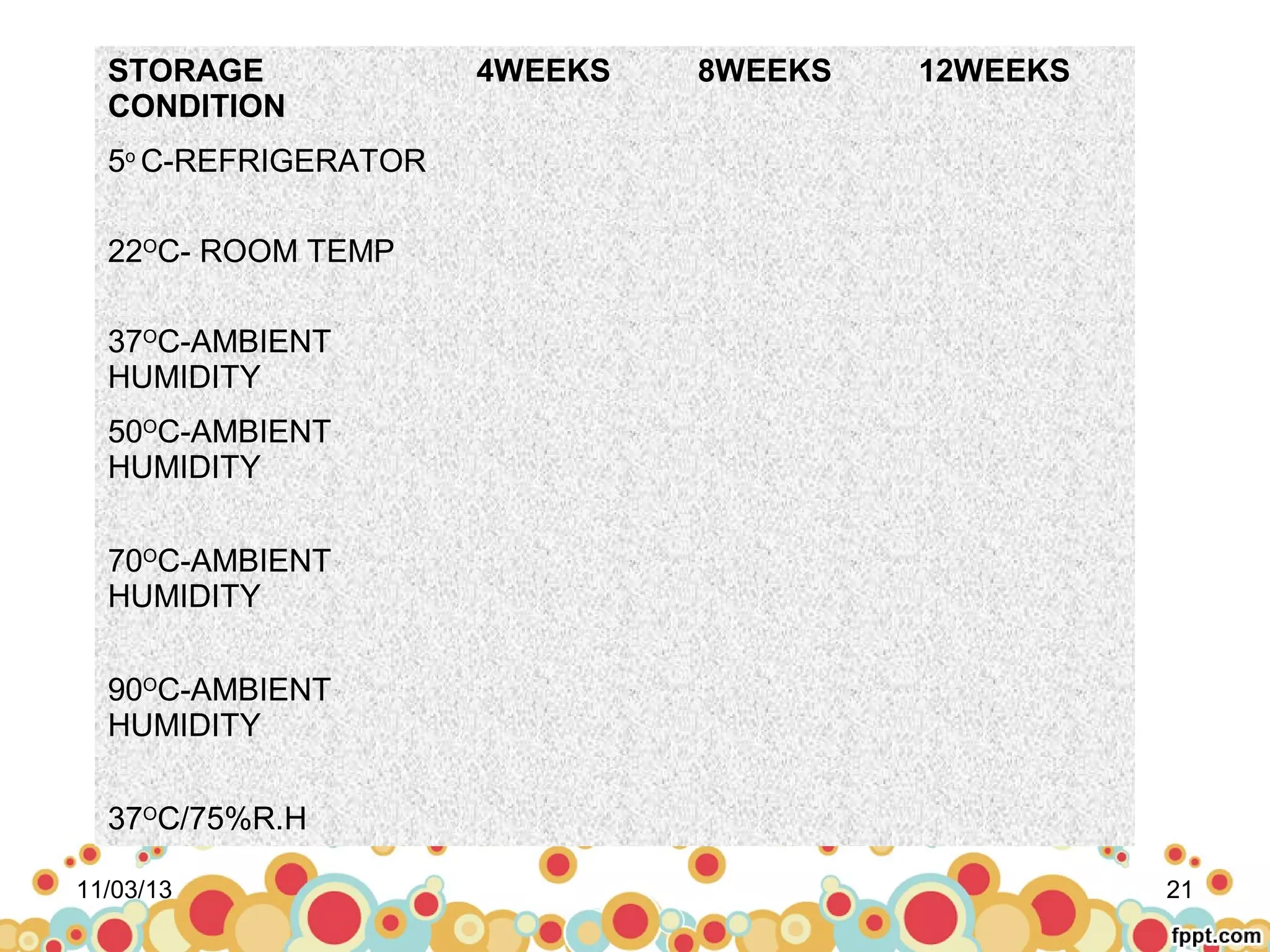
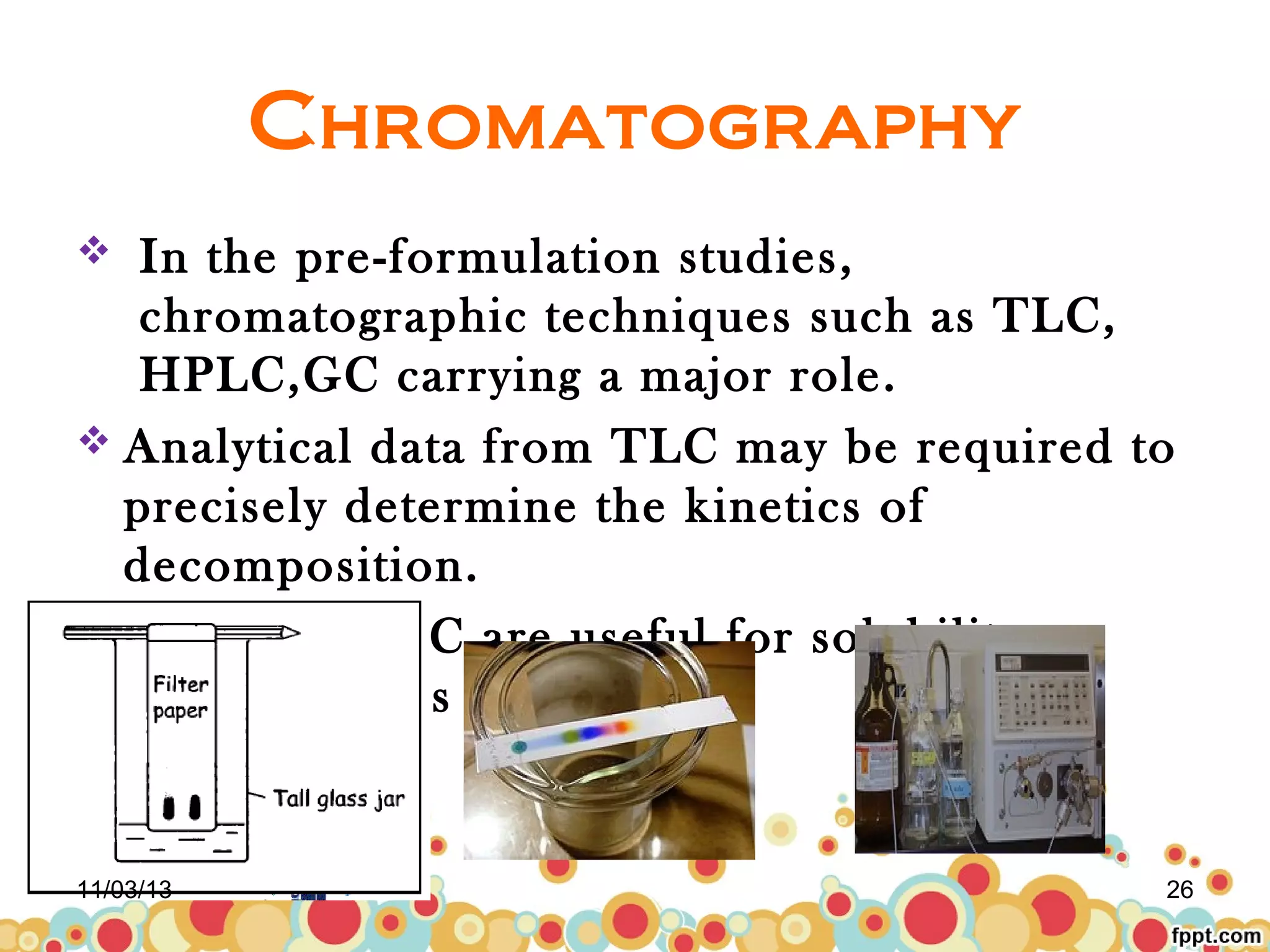
This document discusses preformulation studies for parenteral products. Preformulation testing provides important information for developing dosage forms and involves determining the physical and chemical properties of drug molecules. Key aspects of preformulation studies discussed include bulk characterization of properties like crystallinity and polymorphism. Solubility analysis looks at aqueous solubility, drug ionization, and partition coefficient. Stability studies evaluate stability in formulations, of solutions, and in the solid state under various temperature and humidity conditions. Additional analytical techniques discussed that are useful in preformulation include spectroscopy, microscopy, and chromatography. Thorough preformulation work lays the foundation for developing efficacious and stable dosage forms.