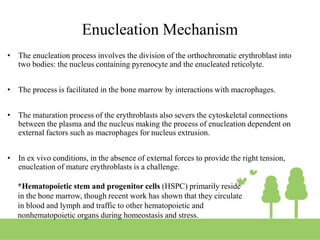
This document discusses stem cell-based approaches for producing red blood cells (RBCs) for transfusion, emphasizing erythropoiesis, challenges in blood compatibility, and the ex vivo generation of RBCs from hematopoietic stem and progenitor cells (HSPCs). It highlights various sources of HSPCs, including umbilical cord blood and induced pluripotent stem cells, and the need for optimized protocols for effective and safe RBC production. The conclusion indicates significant advancements in this research area, with hopes that in vitro generated RBCs will soon complement traditional blood donations.





![EX VIVO PRODUCTION OF HUMAN
RBC
• HSPCs are differentiated into the erythroid lineage by following three steps:
commitment, expansion, and maturation.
• To induce erythroid differentiation and maturation, various cytokines (most of
them include stem cell factor (SCF) and erythropoietin [EPO]) are added to the
culture medium and/or cells are cocultured with feeder cells of murine or human
origin.
• Stem cell factor is a cytokine that binds to the c-KIT receptor.
• A single unit of packed red cells for transfusion contains approximately 2-3 10^12
RBCs.
• We can only isolate 2.5-3 x10^6 to 20.3 x10^6 nucleated cells from 1 ml of
marrow harvested, of which approximately1.5% are CD34+ HSPCs.
* CD34 is a transmembrane phosphoglycoprotein
encoded by the CD34 gene in humans, mice, rats and
other species.](https://image.slidesharecdn.com/ppt-221105050237-97ea3282/85/ppt-pptx-8-320.jpg)



![Primary HSPC's :Source 1
• PB can provide circulating HSPCs in lower numbers, which can be increased by
mobilization using granulocyte colony-stimulating factor. CB provides an enriched
source of CD34+ HSPCs but the total and CD34+ cell number is limited.
• the generation of erythrocytes from CB HSPCs, but ex vivo enucleation from the
cultured cells appeared low (4%) [42]. Nonetheless, these cells were capable of
enucleation in a mouse model.
• Subsequently, the group achieved near-complete (90%) ex vivo terminal maturation of
erythroblasts (derived from CB and PB HSPCs) by coculturing them with mouse MS-5
cells.
• *MS-5cells generate mature T-cells from human hematopoietic stem and
progenitor cells.](https://image.slidesharecdn.com/ppt-221105050237-97ea3282/85/ppt-pptx-12-320.jpg)





