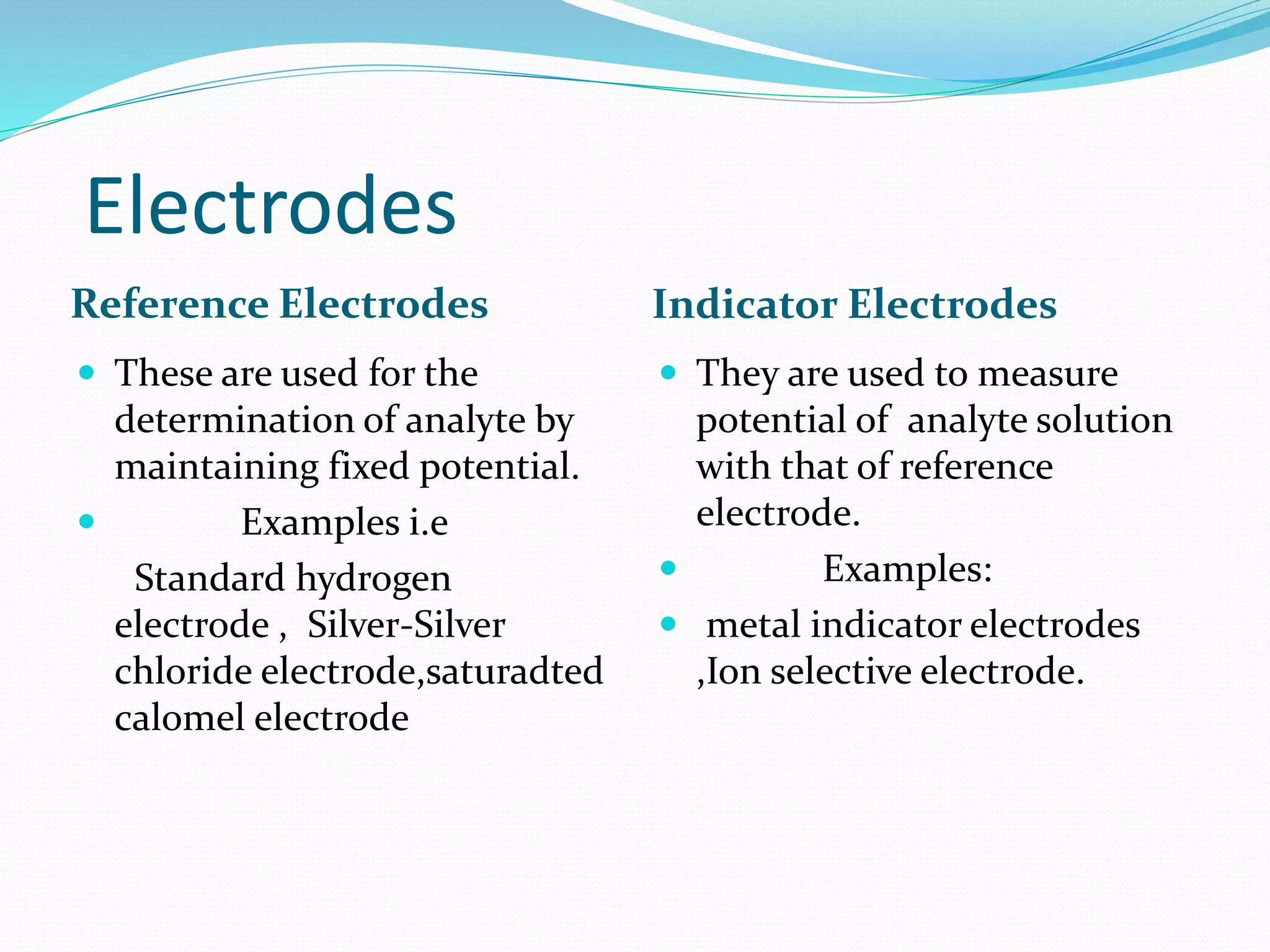
This document provides an introduction to electrochemical methods. It discusses that electrochemistry concerns the interaction of electrical and chemical effects. Five major electrochemical methods are described: potentiometry, conductometry, dielectrometry, voltammetry, and coulometry. Potentiometry involves measuring the potential between two electrodes using a high impedance voltmeter. Ion selective electrodes are discussed in detail, including their types, construction, advantages, limitations, and applications. Other topics covered include electrochemical cells, electrode potentials, the Nernst equation, electrochemical sensors, and potentiometry instrumentation and applications.