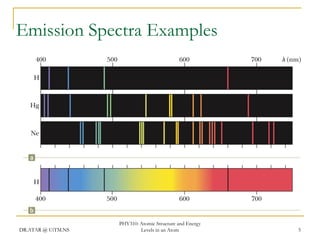
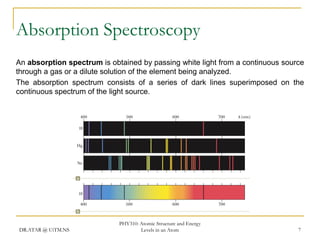
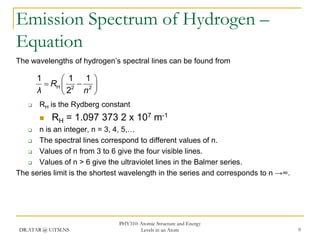
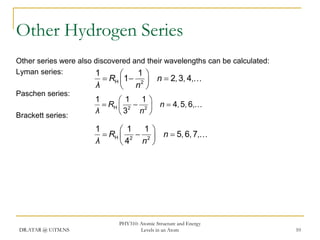
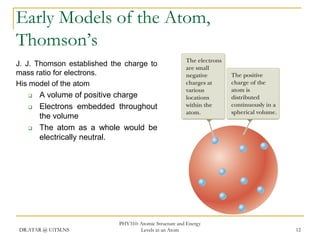
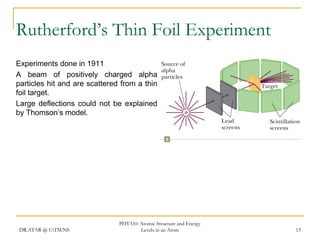
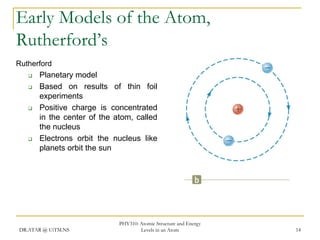
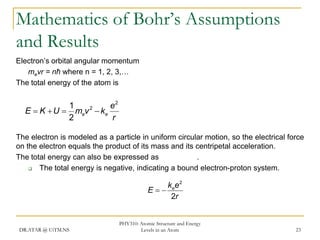
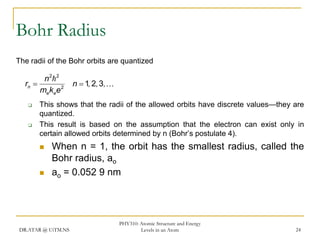
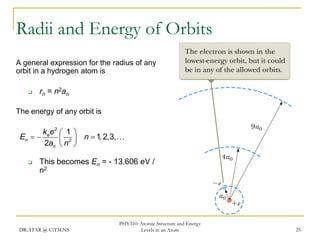
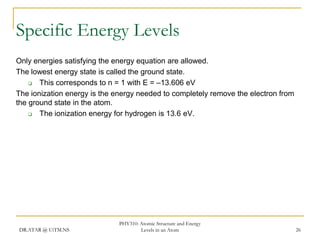
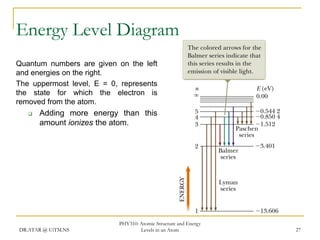
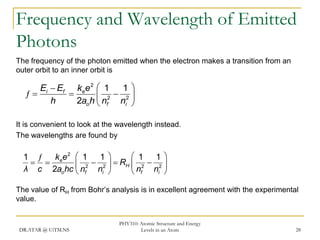
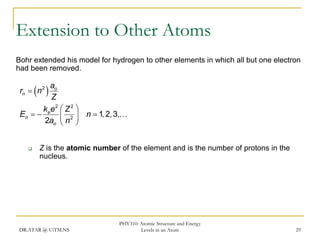
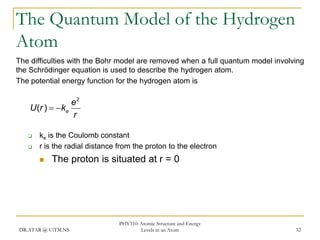
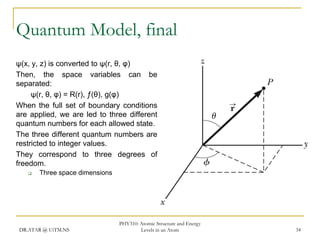
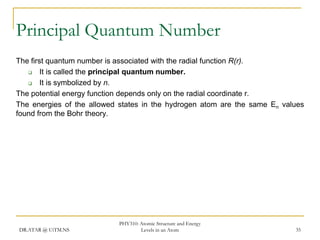
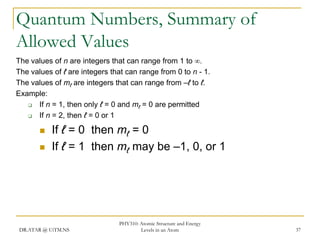
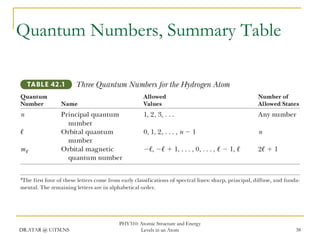
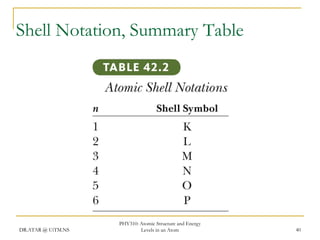
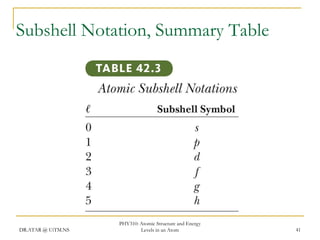
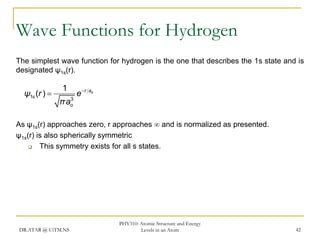
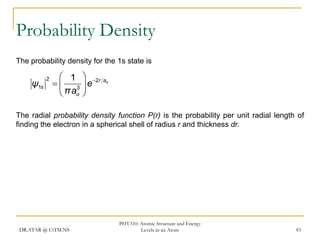
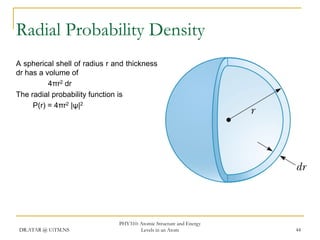
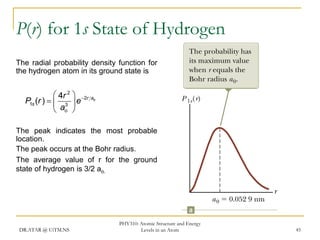
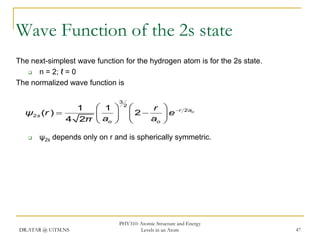
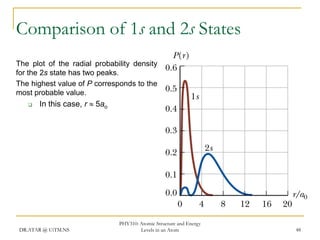
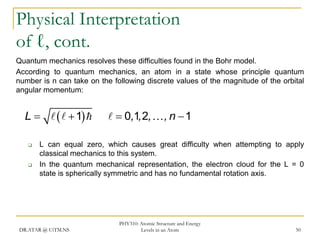
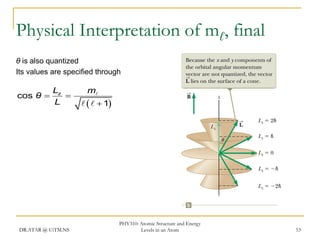
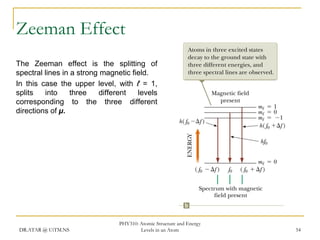
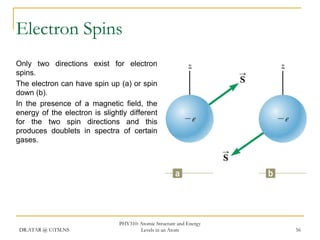
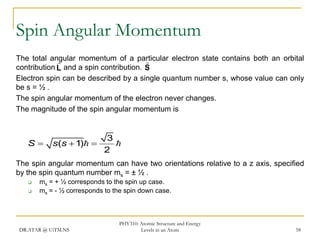
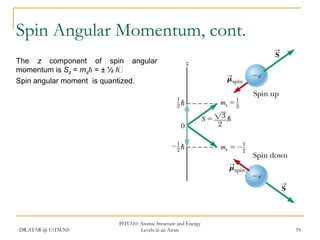
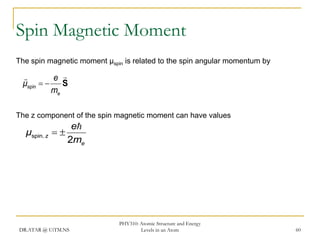
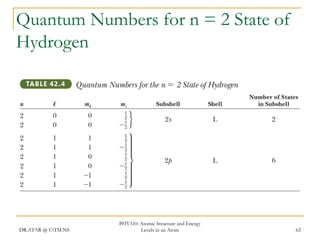
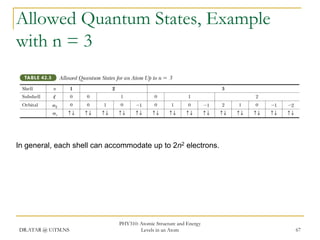
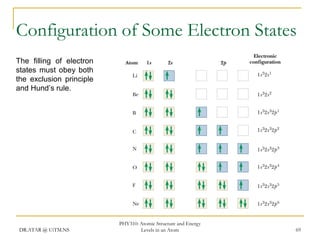
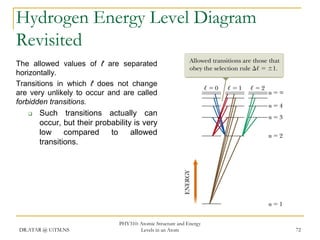
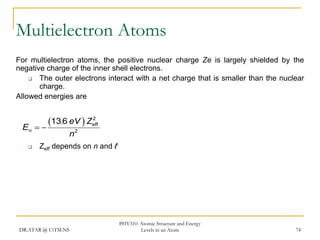
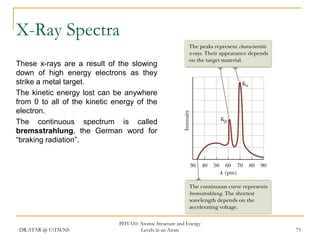
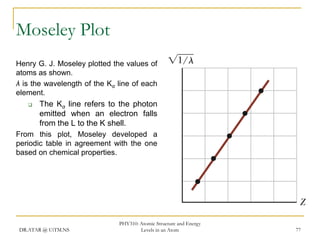
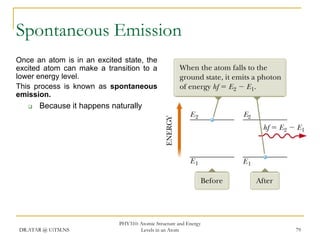
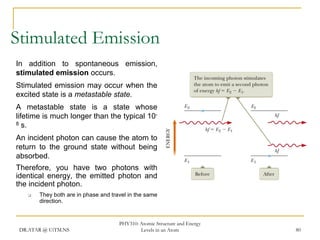
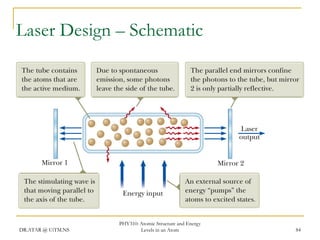
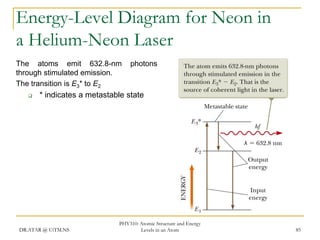
The document discusses atomic structure and energy levels in atoms. It begins by focusing on the importance of the hydrogen atom in understanding atomic physics. The hydrogen atom can be solved exactly and its properties extended to other atoms. Its spectra allow for precision tests of theory. Later models like the Rutherford model and Bohr model improved upon the early "plum pudding" model. Bohr's model combined classical mechanics with Planck's idea of quantized energy levels to explain the discrete emission spectra of atoms. His four postulates introduced new ideas like stationary, quantized electron states that allowed atoms to retain energy.