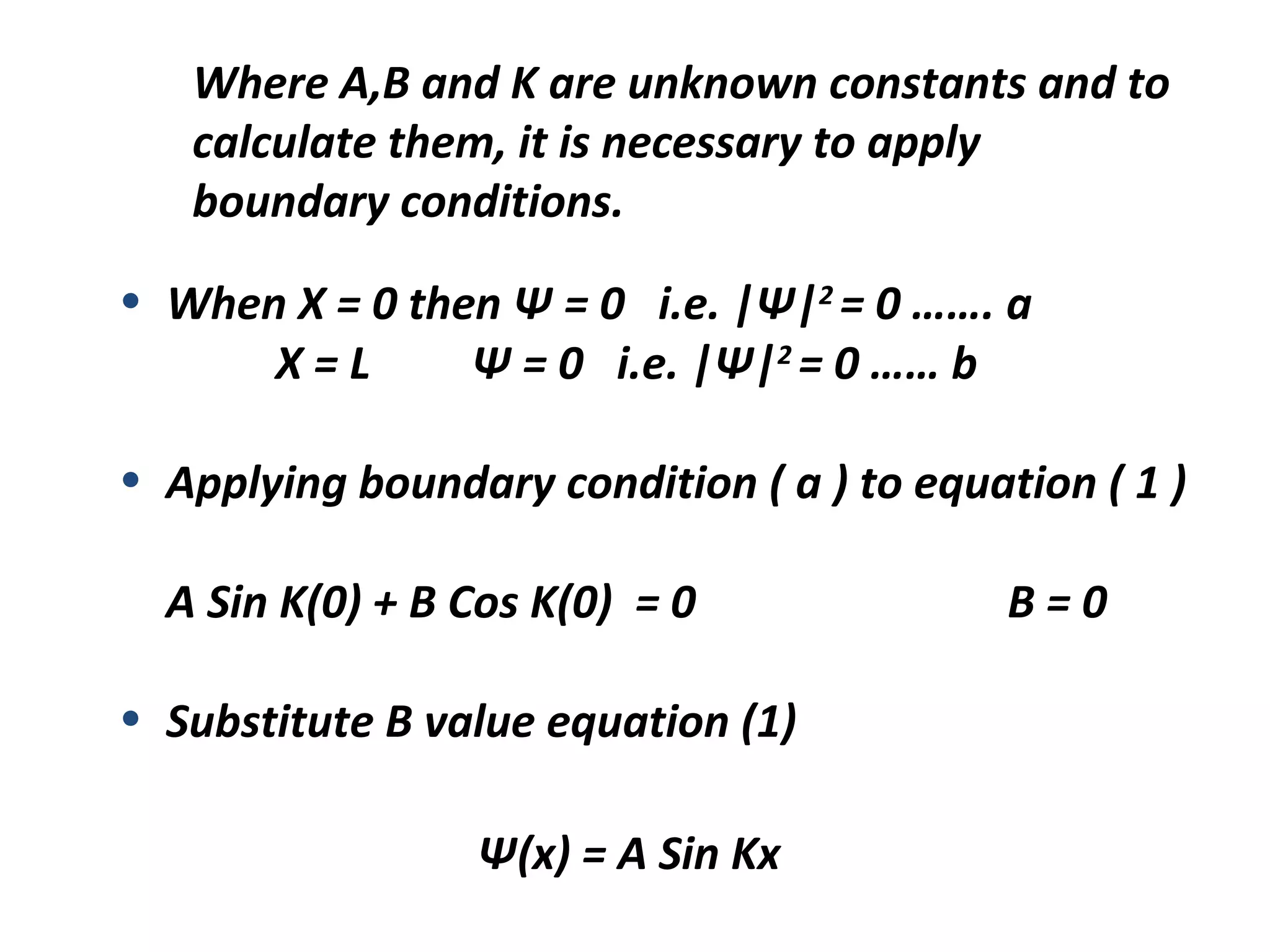
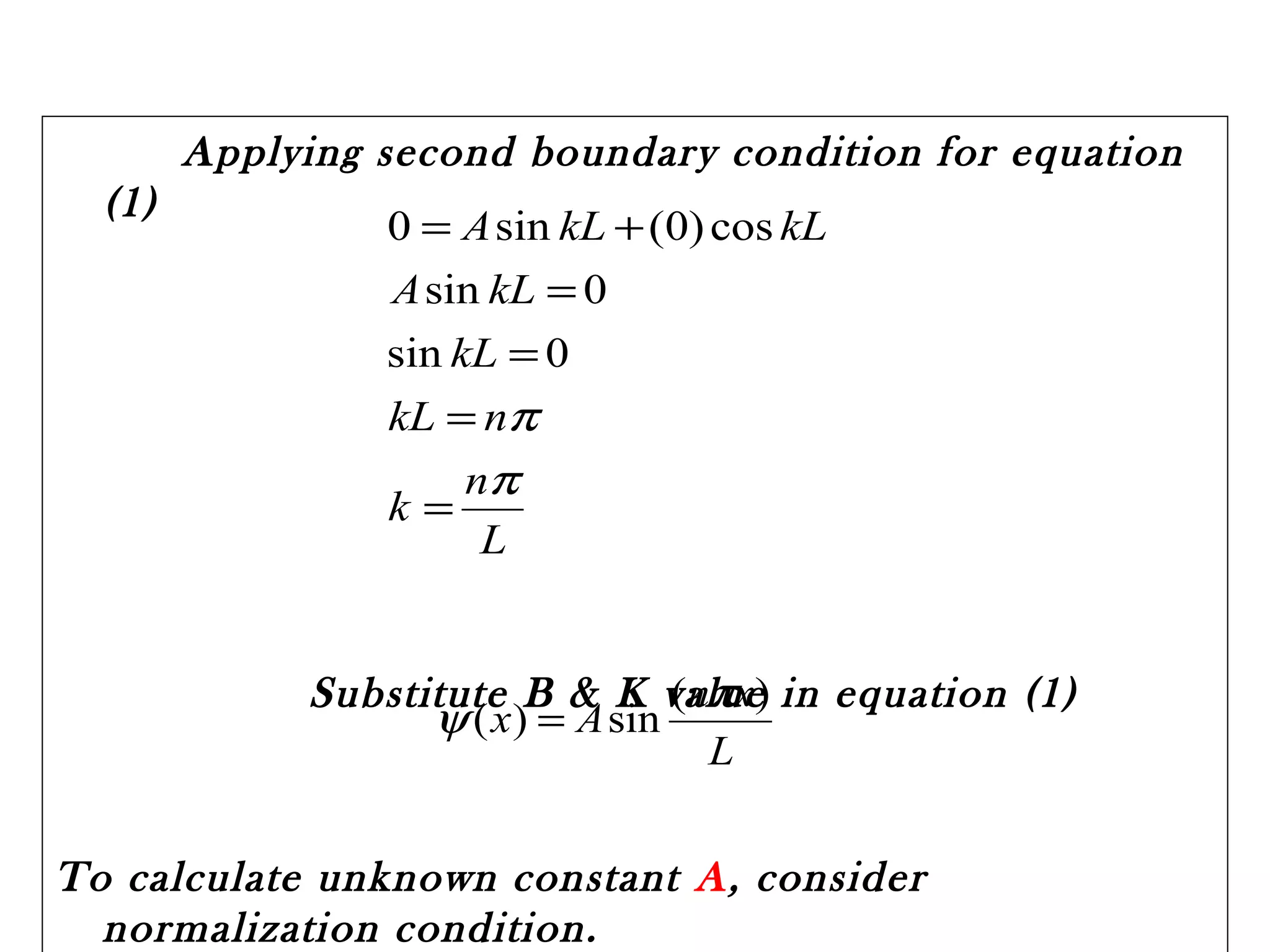
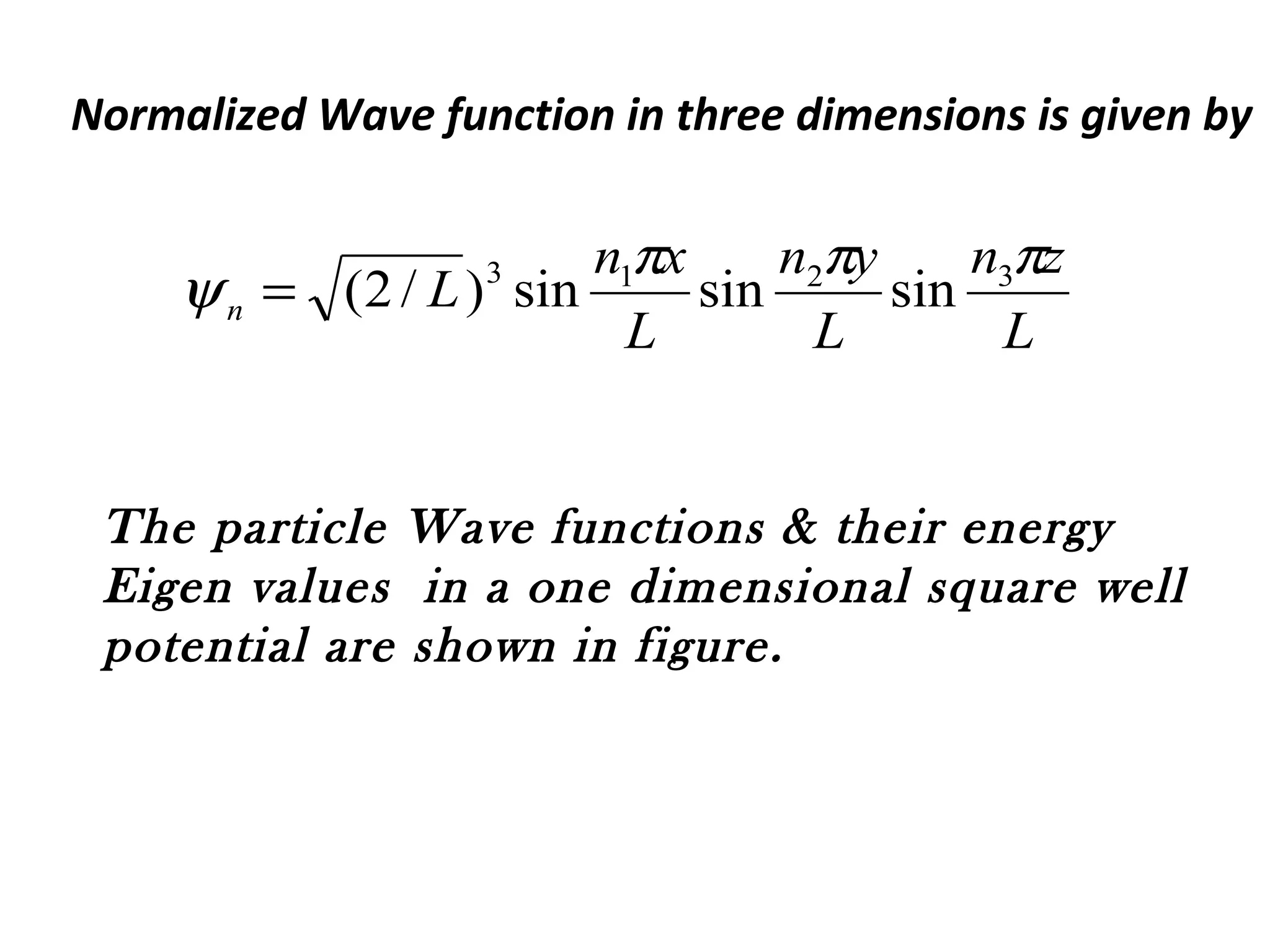
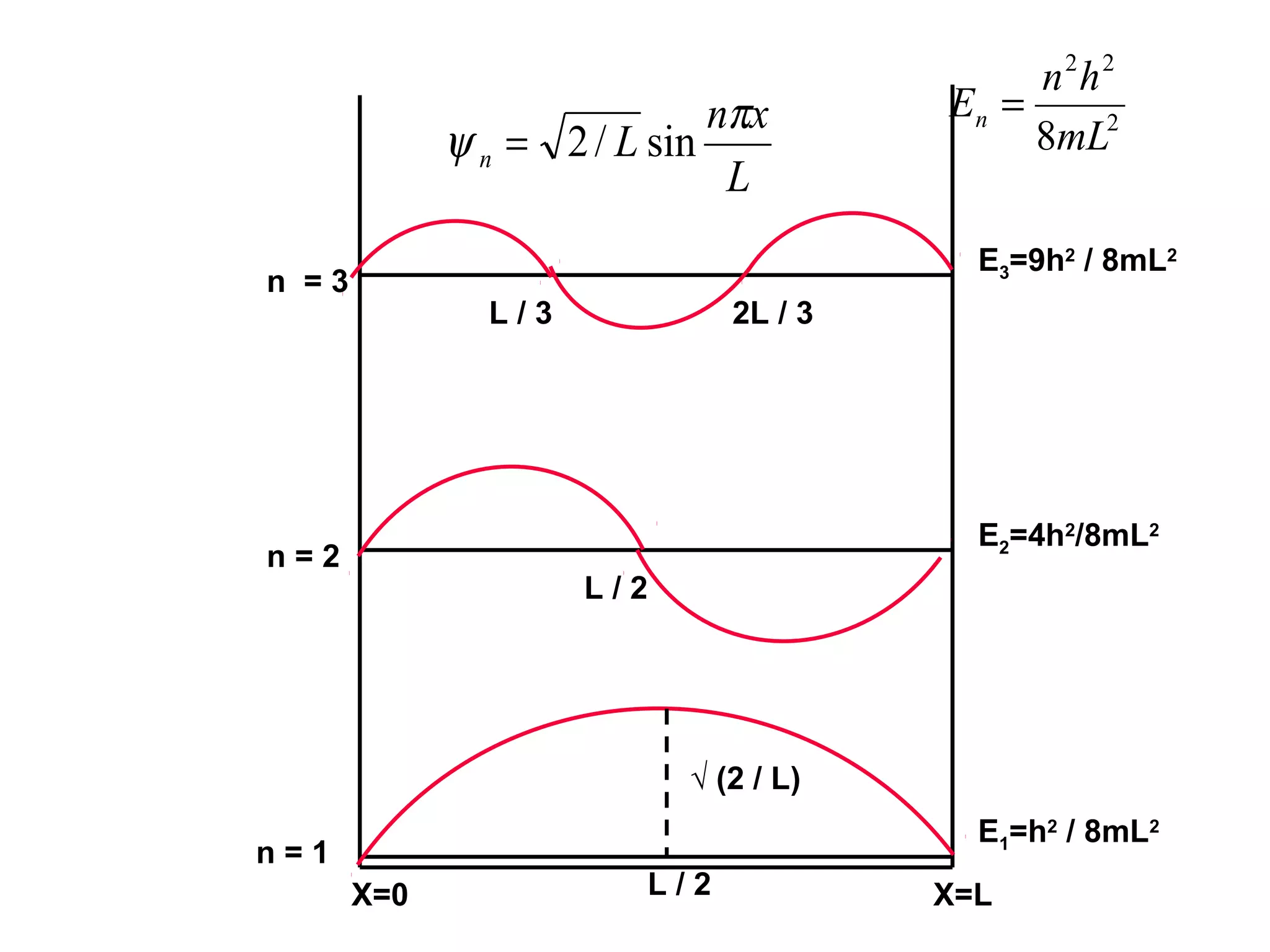
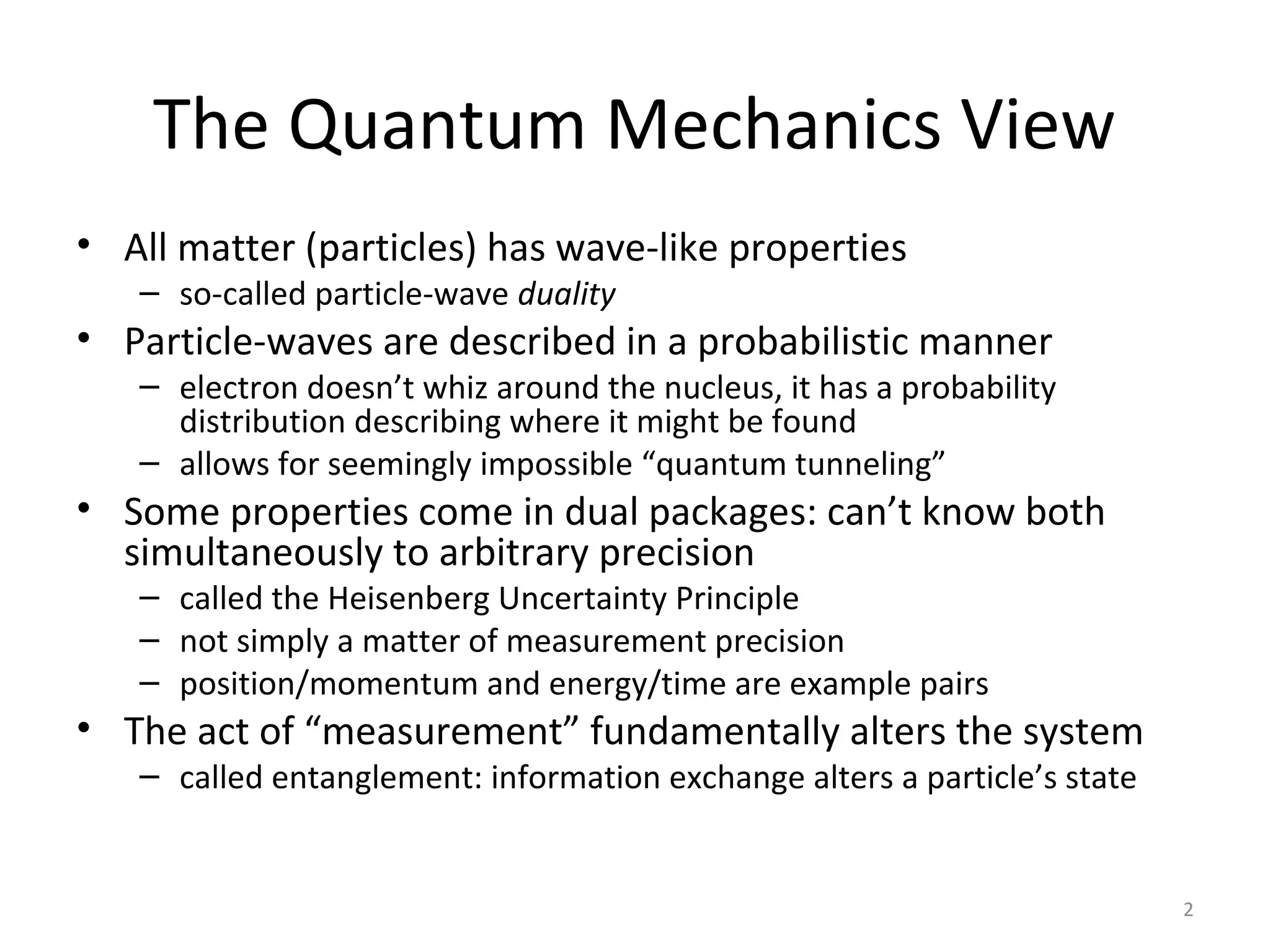
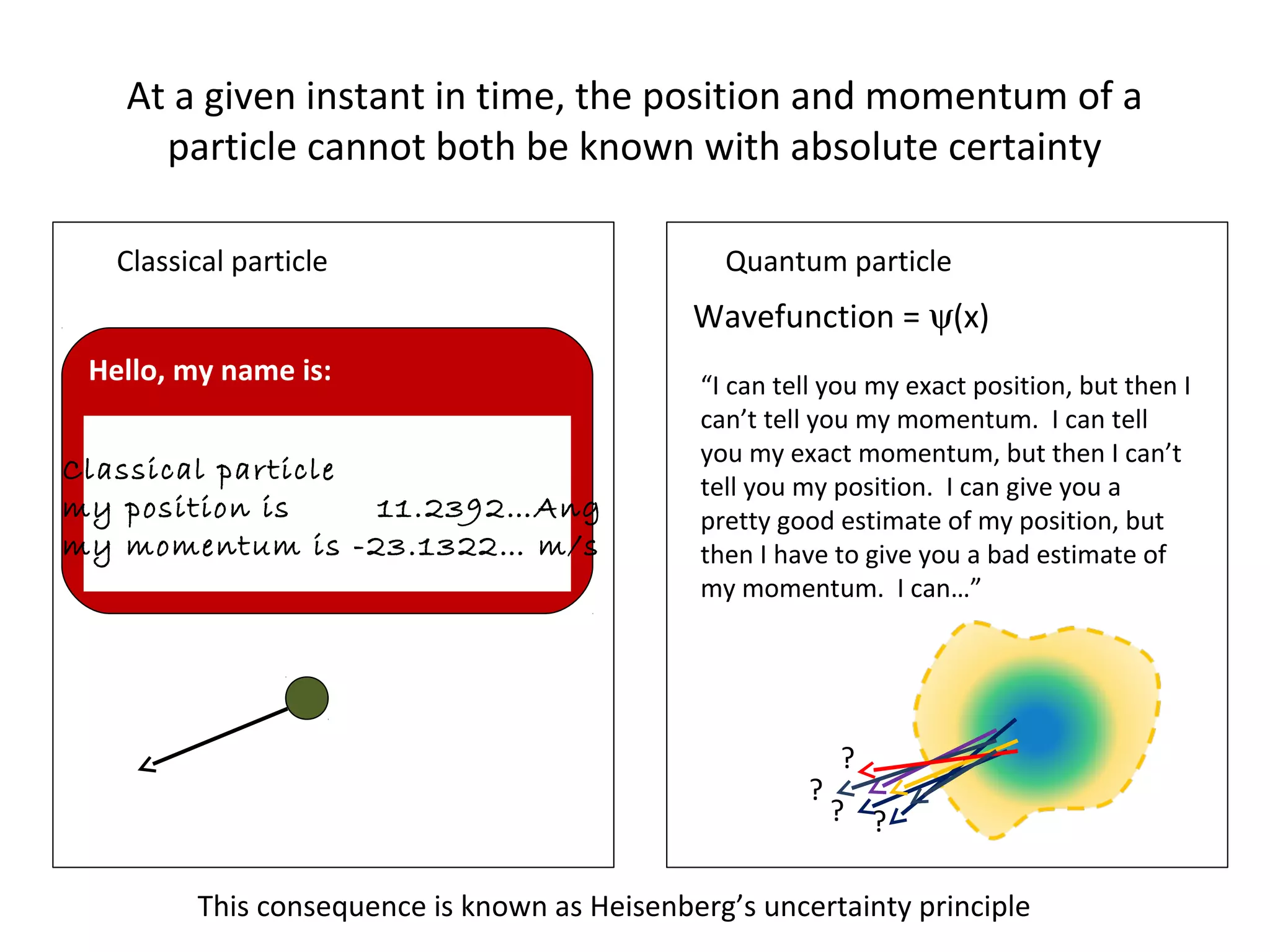
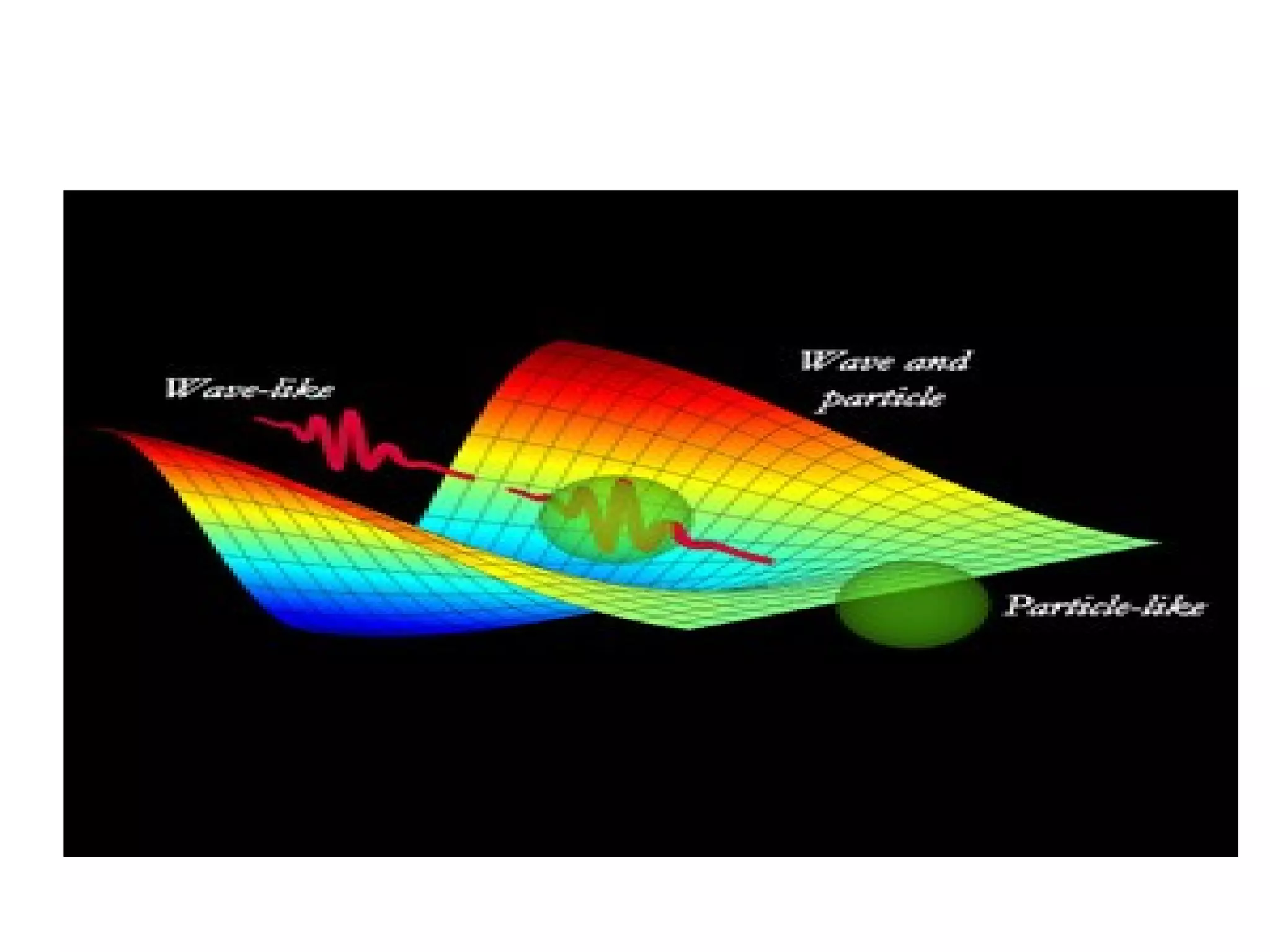
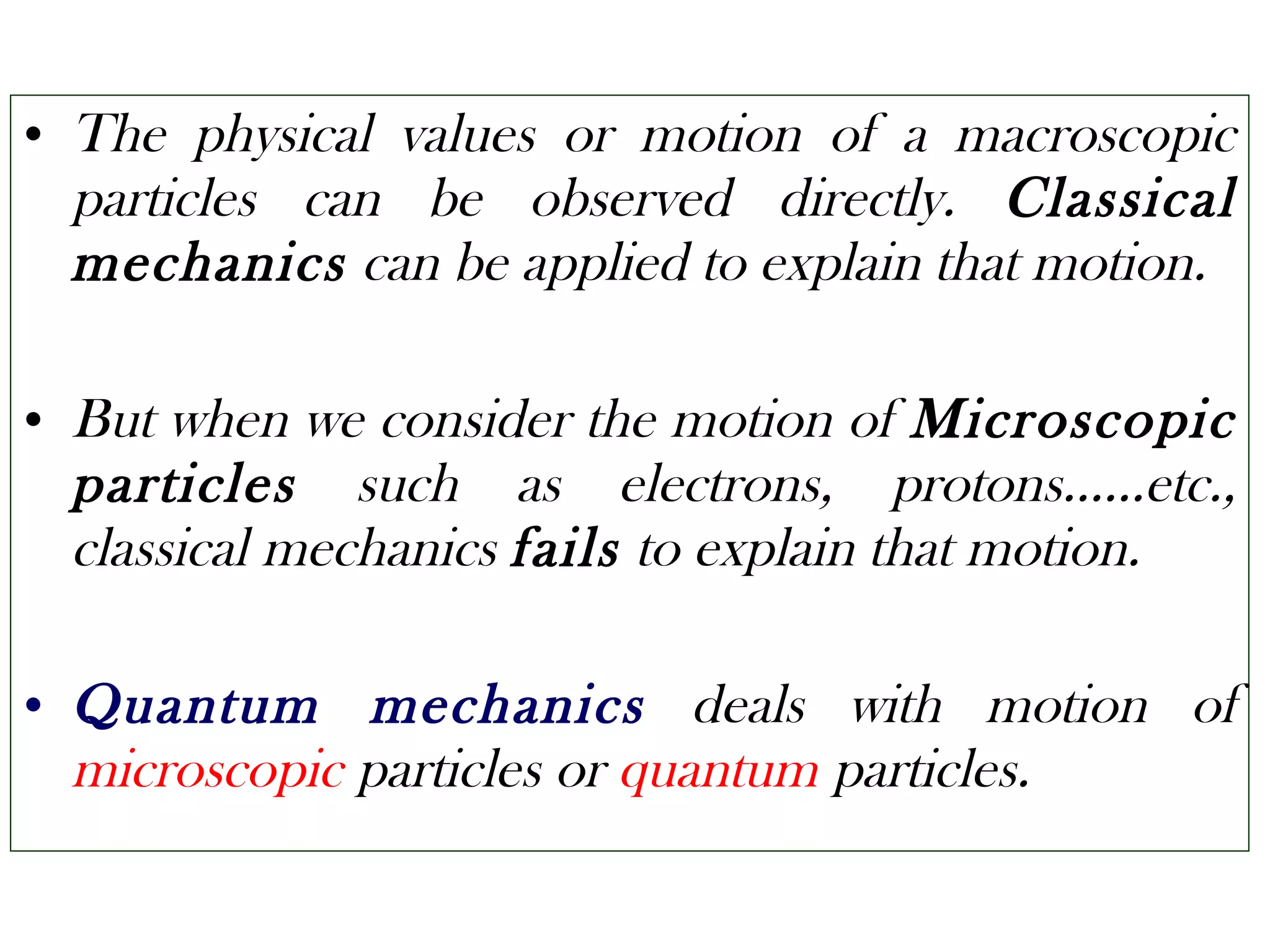
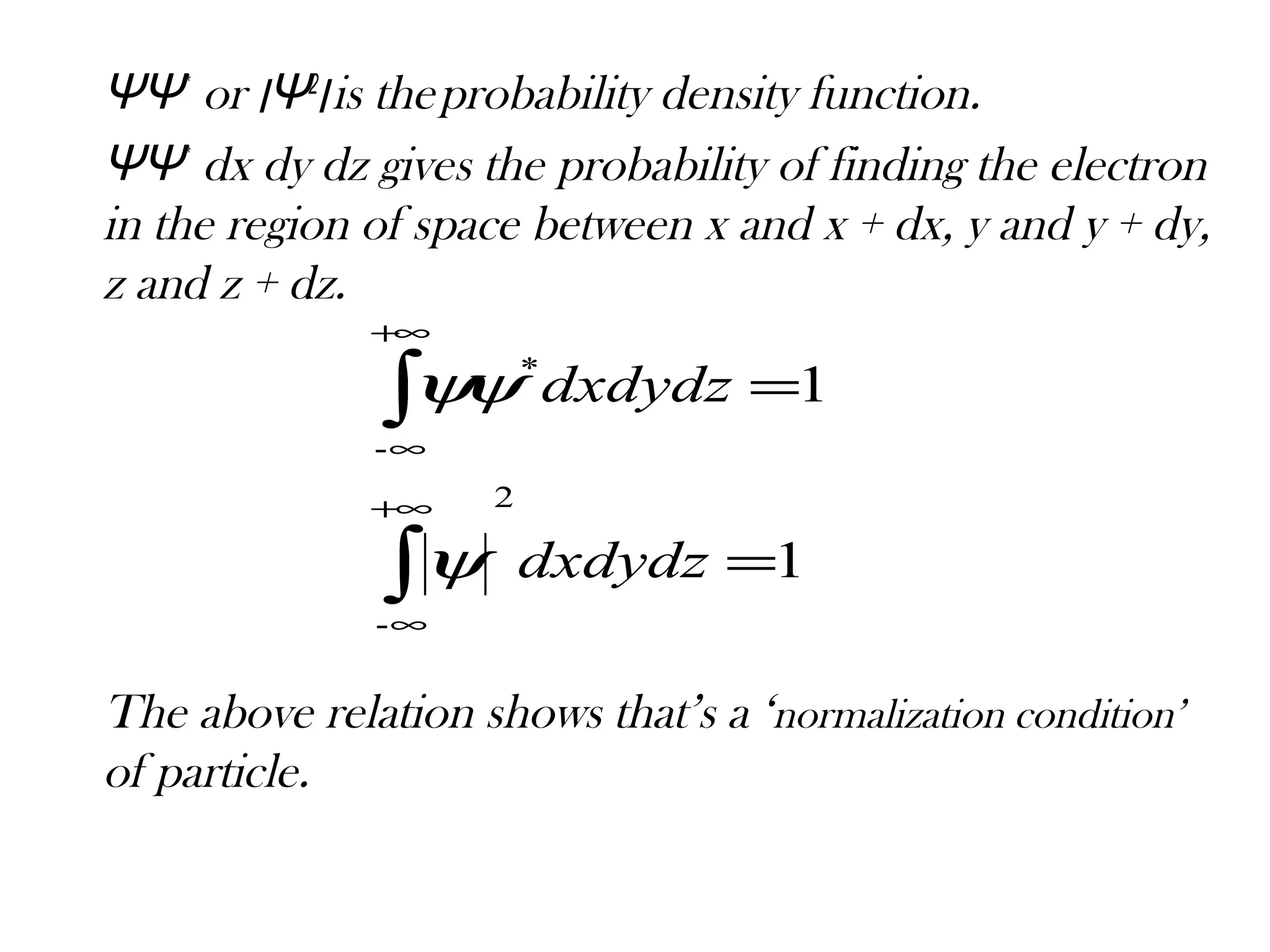
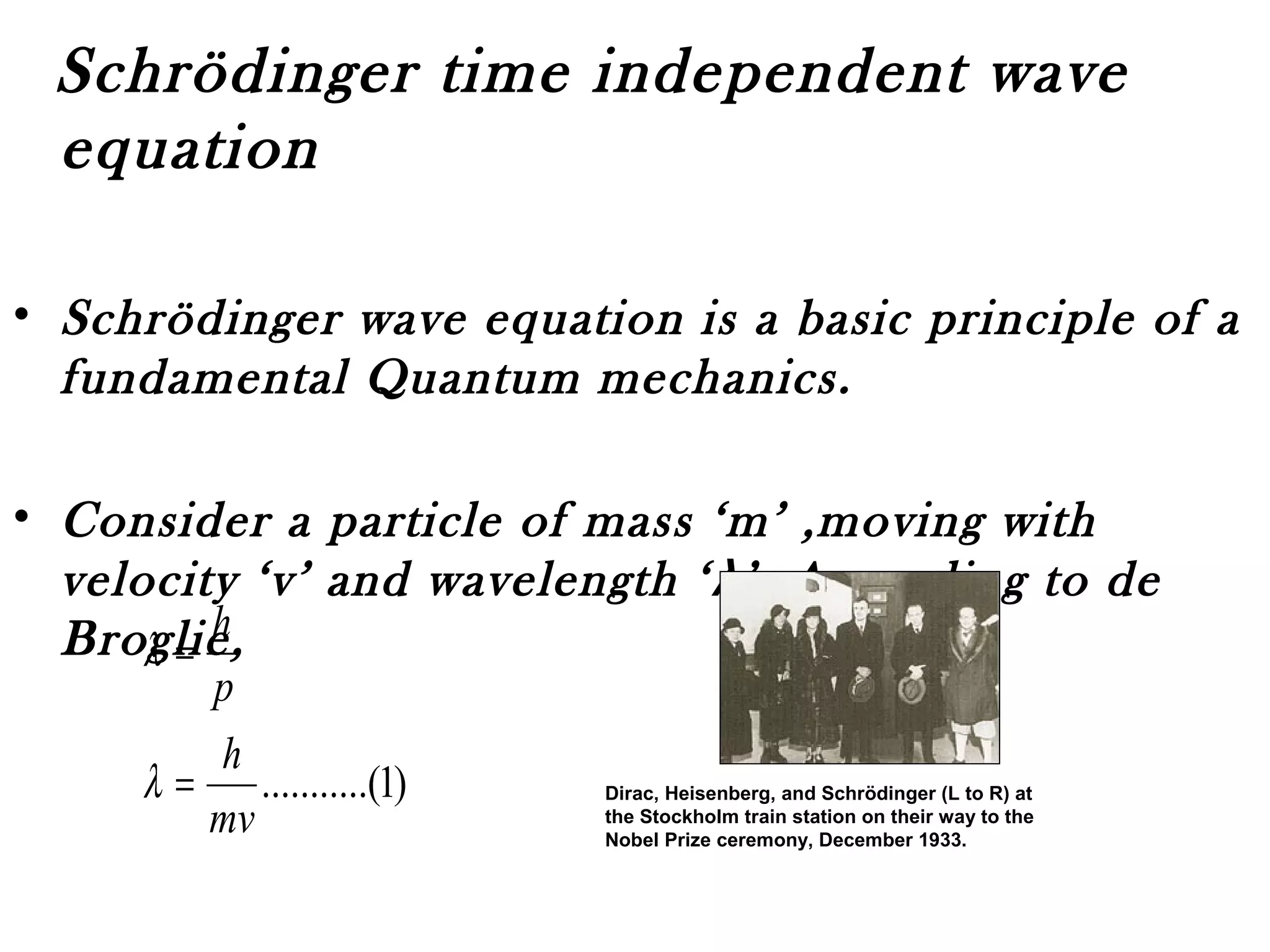
Quantum mechanics describes the behavior of matter and light on the atomic and subatomic scale. Some key points of the quantum mechanics view are that particles can exhibit both wave-like and particle-like properties, their behavior is probabilistic rather than definite, and some properties like position and momentum cannot be known simultaneously with complete precision due to the Heisenberg uncertainty principle. Quantum mechanics has successfully explained various phenomena that classical physics could not and led to important technologies like lasers, MRI machines, and semiconductor devices.















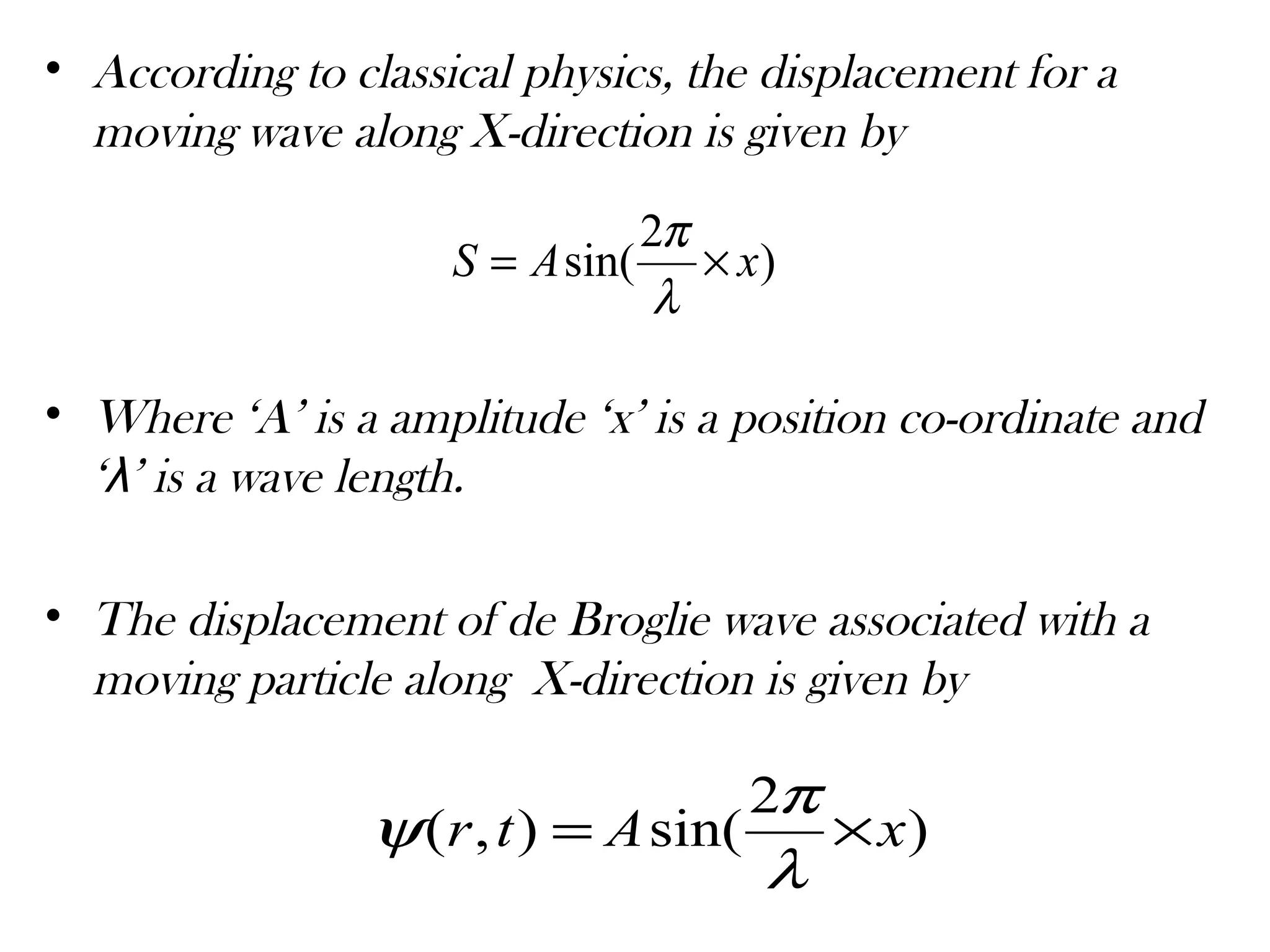
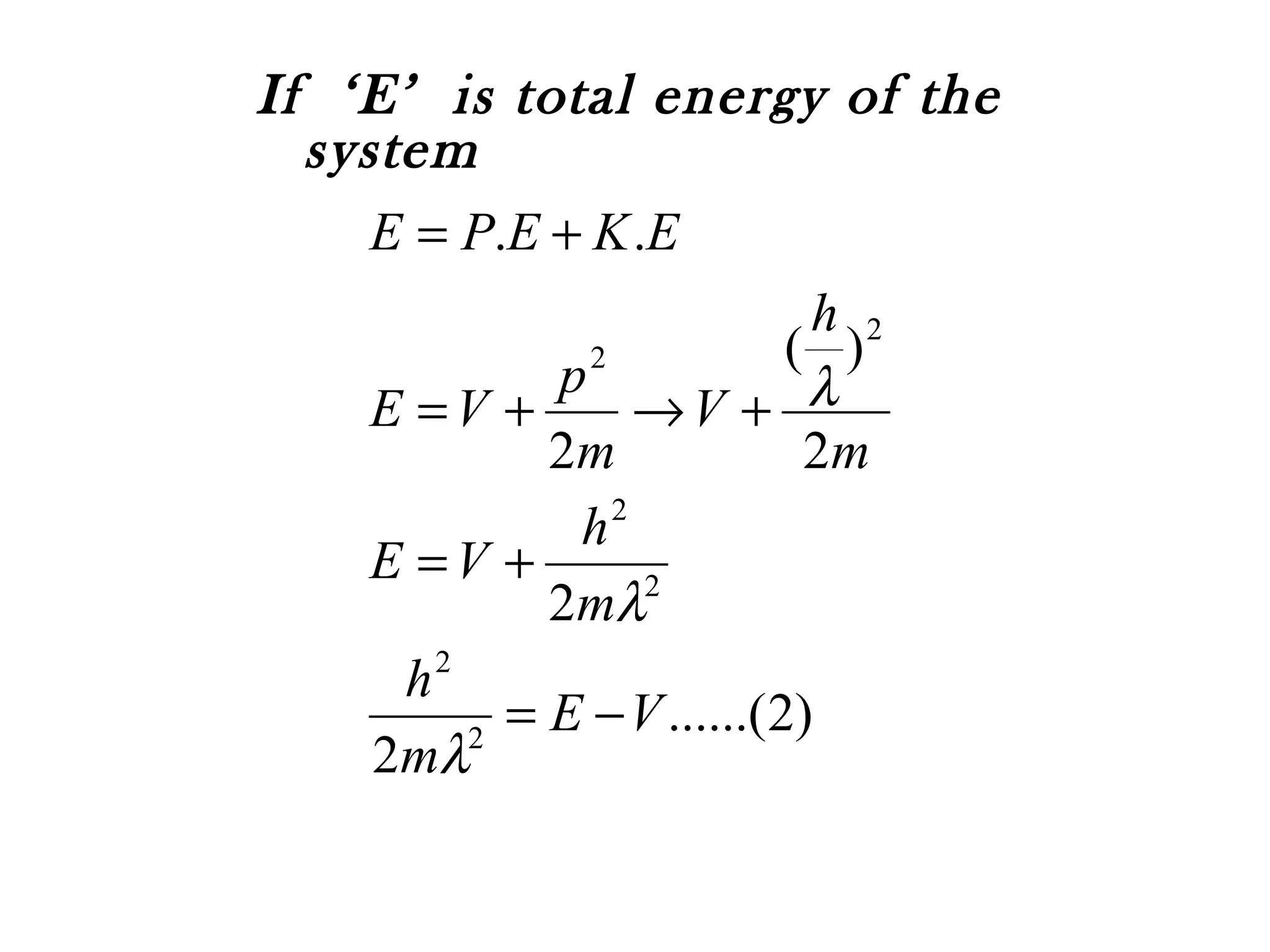
![Periodic changes in ‘Ψ’ are responsible for the
wave nature of a moving particle
)3(
4
11
4
).
2
sin(
4
).
2
sin(]
2
[
).
2
cos(
2)(
].
2
sin[
)(
2
2
22
2
2
2
2
2
2
2
2
2
2
2
→−=
−=
−=
−=
=
=
dx
d
dx
d
xA
dx
d
xA
dx
d
xA
dx
d
xA
dx
d
dx
d
ψ
ψπλ
ψ
λ
πψ
λ
π
λ
πψ
λ
π
λ
πψ
λ
π
λ
πψ
λ
πψ](https://image.slidesharecdn.com/quantummechanics-130531050629-phpapp01/75/Quantum-mechanics-a-brief-53-2048.jpg)
![0][
8
][
8
][
8
][]
4
1
[
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
2
=−+
−−=
−=
−
−=−
−=
ψ
πψ
ψ
πψ
ψ
ψπ
ψ
ψπ
λ
VE
h
m
dx
d
VE
h
m
dx
d
VE
dx
d
m
h
VE
dx
d
m
h
VE
m
h
This is Schrödinger time independent wave equation in one dimension.
From equation 3……
0][
8
2
2
2
2
2
2
2
2
=−+
∂
∂
+
∂
∂
+
∂
∂
ψ
πψψψ
VE
h
m
zyx
In three dimensional way it becomes…..](https://image.slidesharecdn.com/quantummechanics-130531050629-phpapp01/75/Quantum-mechanics-a-brief-54-2048.jpg)
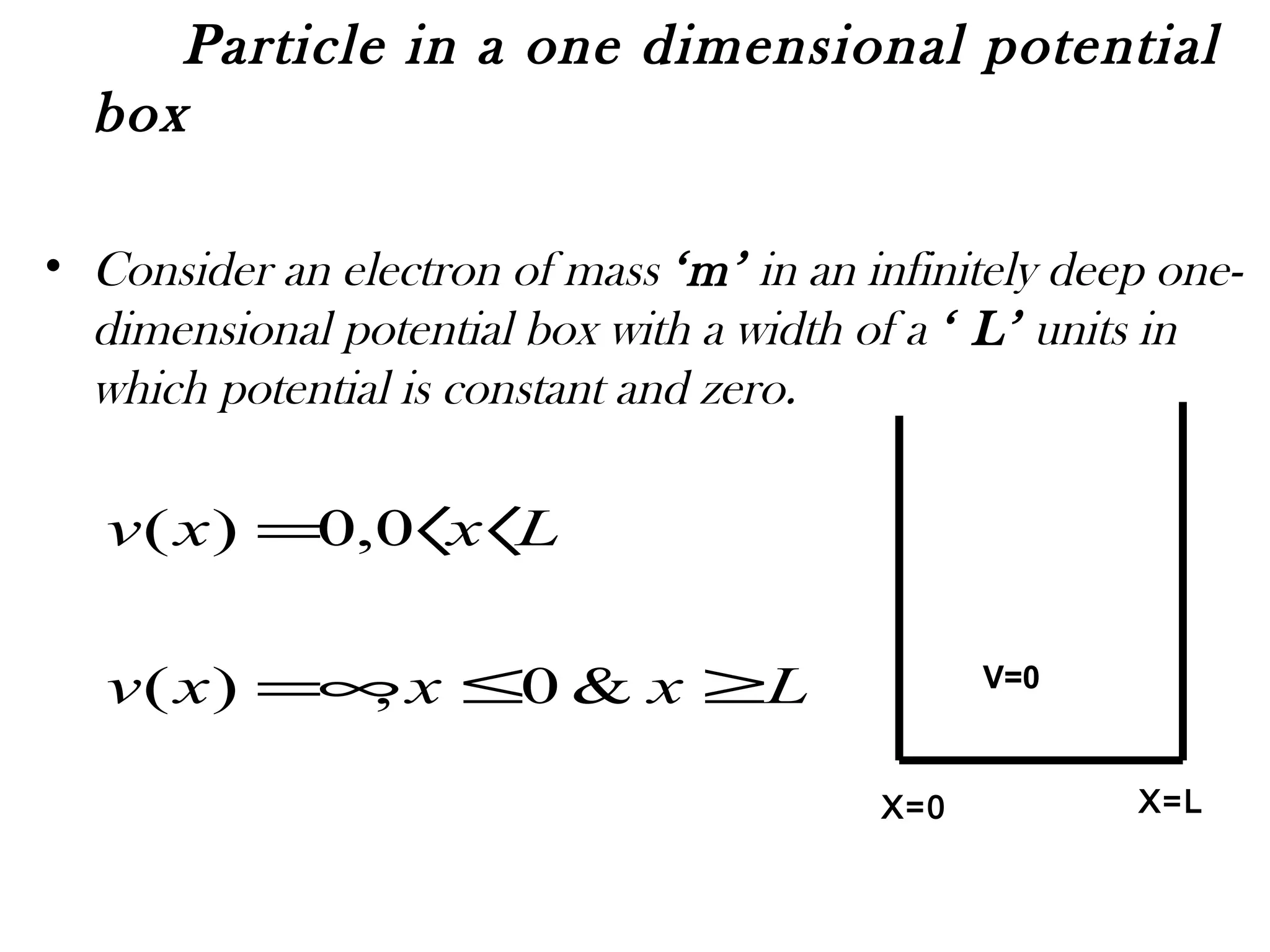
![The motion of the electron in one dimensional
box can be described by the Schrödinger's
equation.
0][
2
22
2
=−+ ψ
ψ
VE
m
dx
d
Inside the box the potential V =0
E
m
kwherek
dx
d
E
m
dx
d
2
22
2
2
22
2
2
,,0
0][
2
=→=+
=+
ψ
ψ
ψ
ψ
kxBkxAx cossin)( +=ψ
The solution to above equation can be written as](https://image.slidesharecdn.com/quantummechanics-130531050629-phpapp01/75/Quantum-mechanics-a-brief-57-2048.jpg)