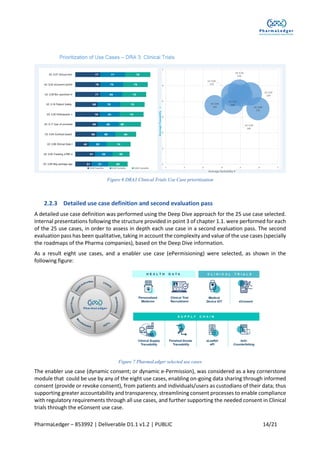
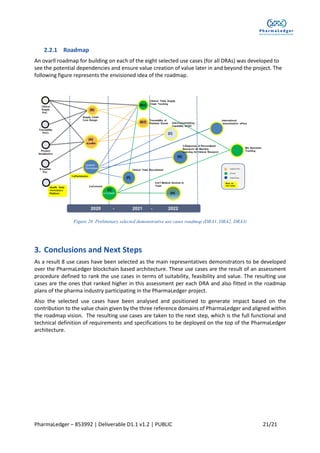
This document outlines the use case prioritization and selection process for the Pharmaledger project, which is funded by the Innovative Medicines Initiative. It details a methodology developed for evaluating over 88 nominated use cases based on value, feasibility, and suitability, resulting in eight selected use cases across supply chain, health data, and clinical trials, with a focus on blockchain technology. The report emphasizes collaboration among various stakeholders to capture end-user needs and create user-driven solutions in the healthcare industry.