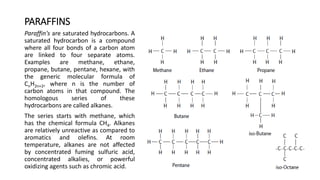
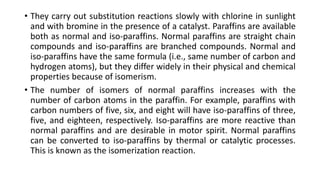
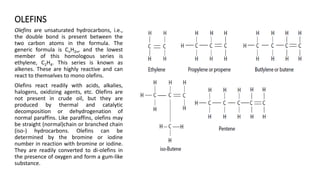
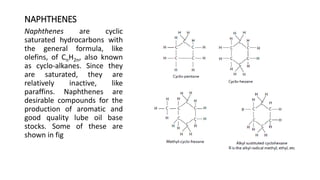
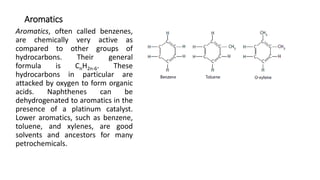
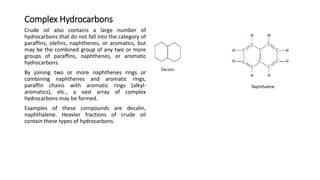
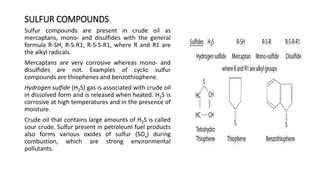
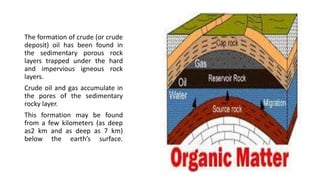
This document provides an overview of petroleum refinery engineering. It discusses the origin and composition of crude oil, refinery processes like distillation, cracking and reforming. It covers auxiliary operations, product design and the use of linear programming. Key terms like petroleum, refining, distillation, cracking and reforming are defined. Recommended books on petroleum refinery technology and economics are also listed.