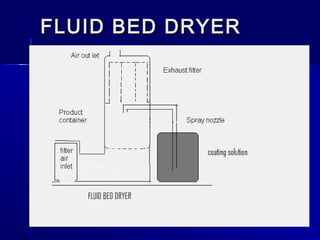
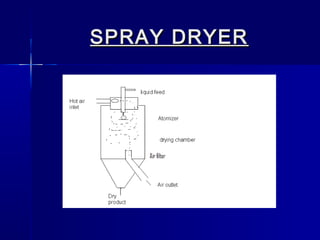
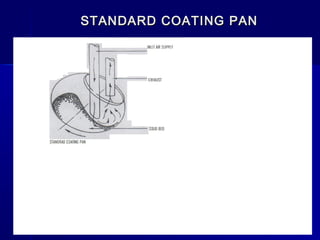
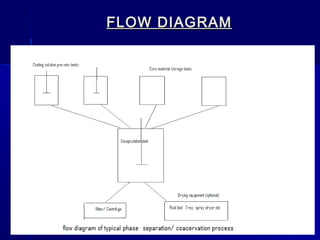
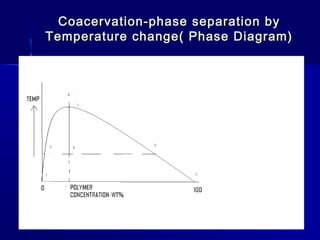
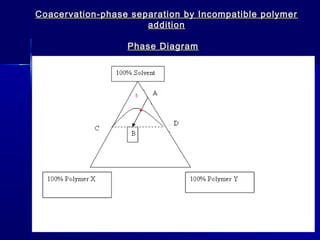
Microencapsulation involves coating small particles or droplets to form microcapsules. It can be used to modify drug release timing and mask tastes. Various coating materials can be used depending on the product requirements. Common microencapsulation methods include spray drying, fluidized bed coating, pan coating, coacervation, and solvent evaporation. These methods use different processes but generally involve applying a coating and hardening it to form microcapsules encapsulating core materials. Microencapsulation offers various applications in pharmaceutical products.