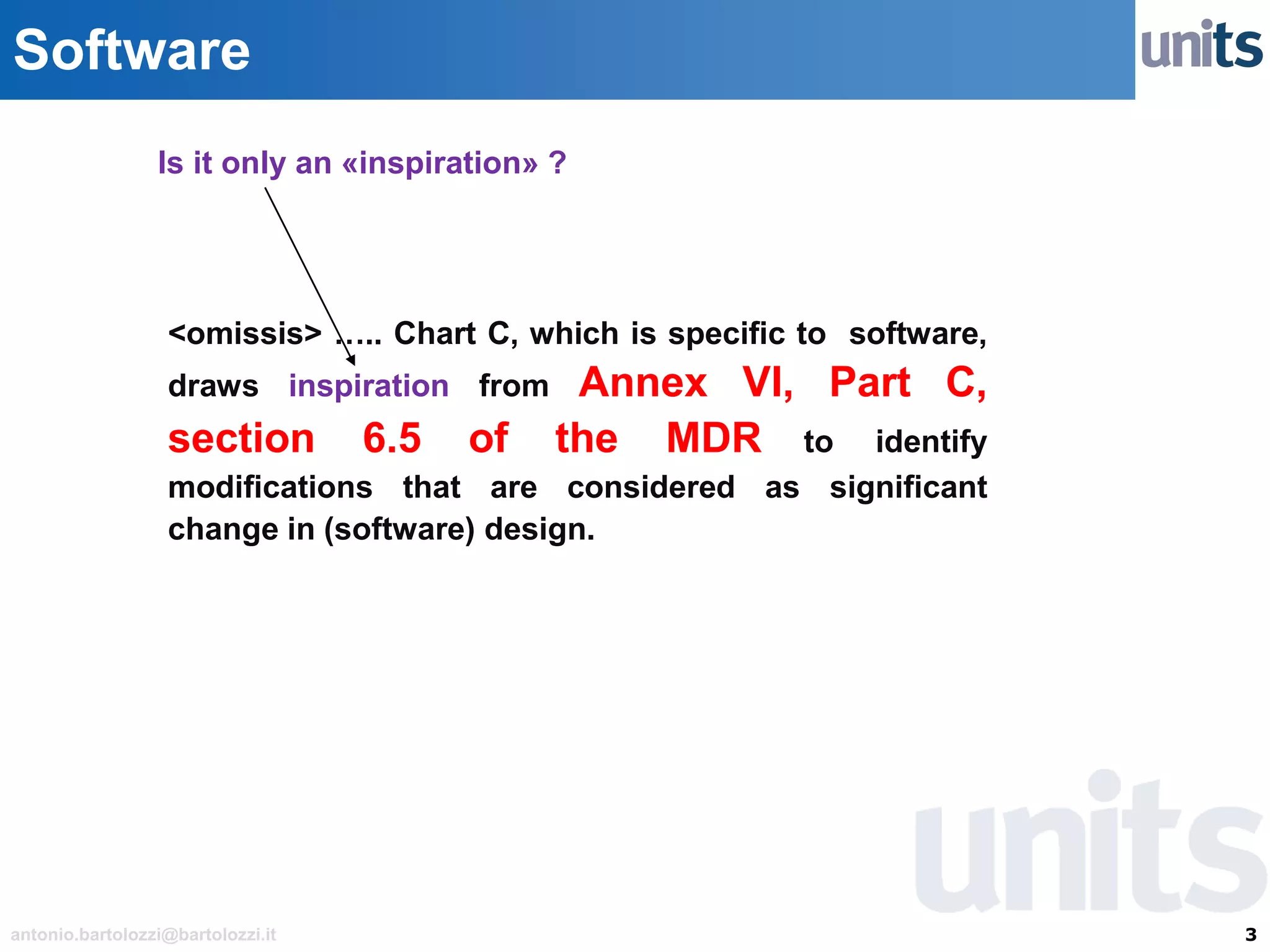
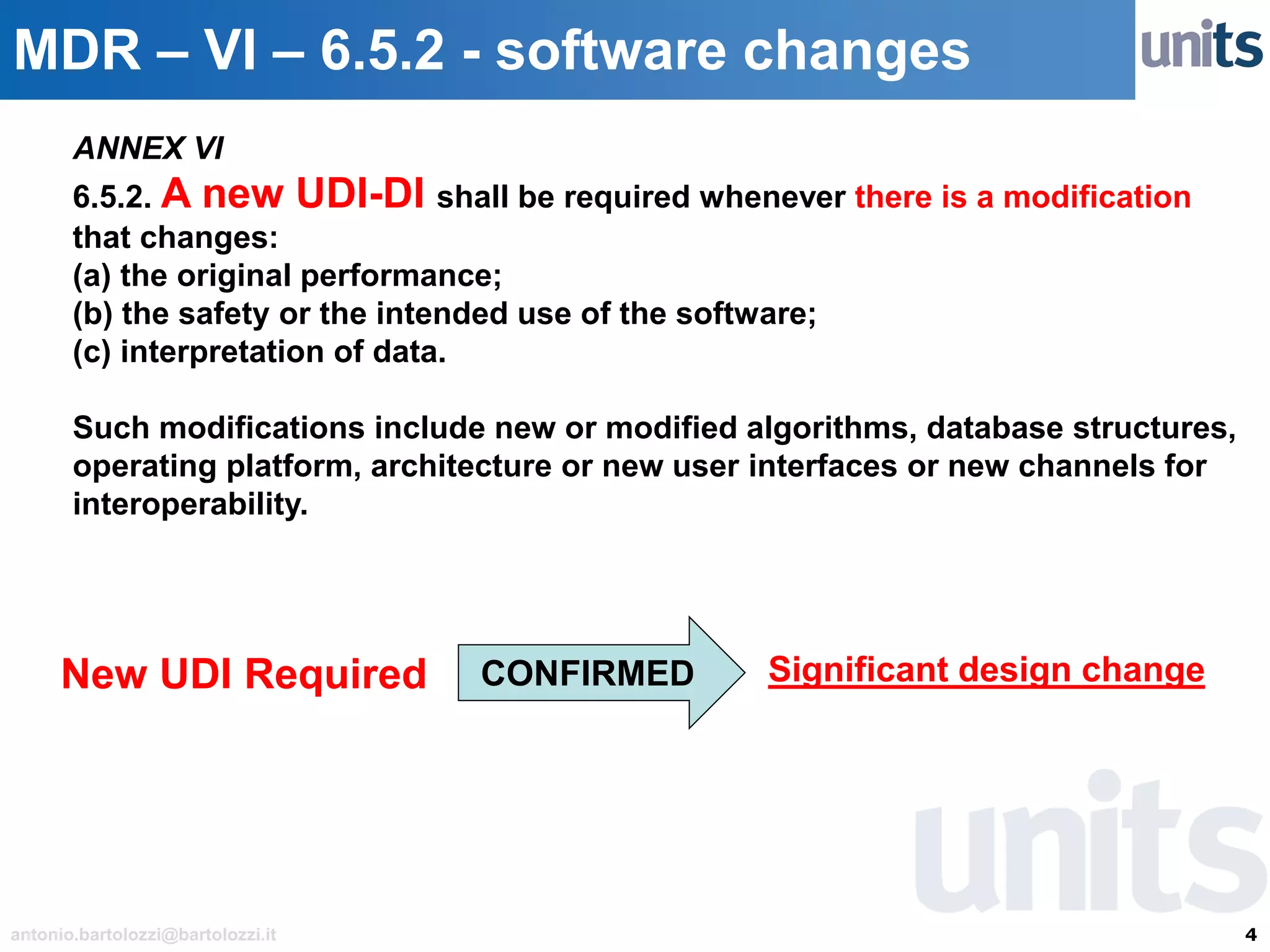
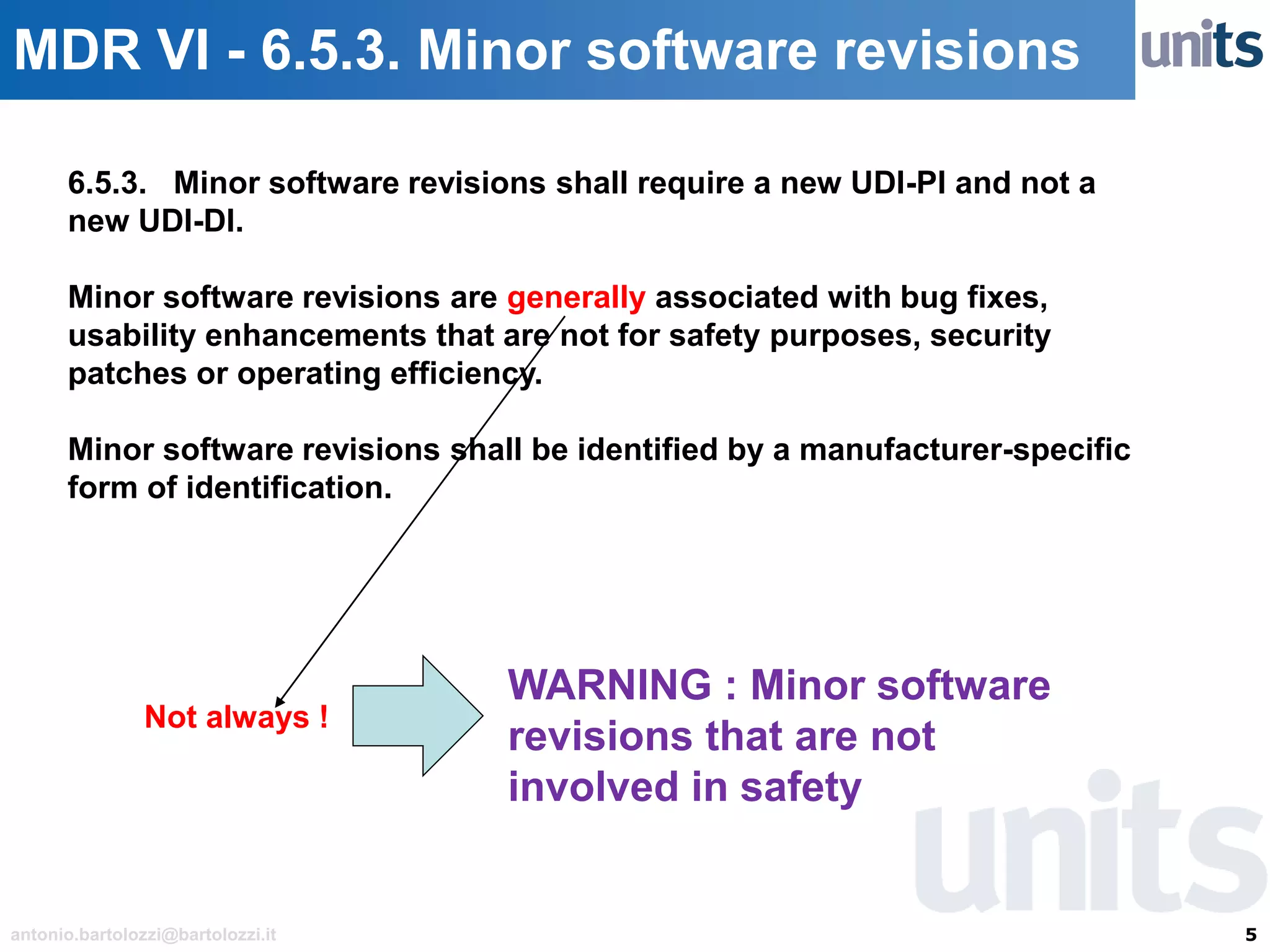
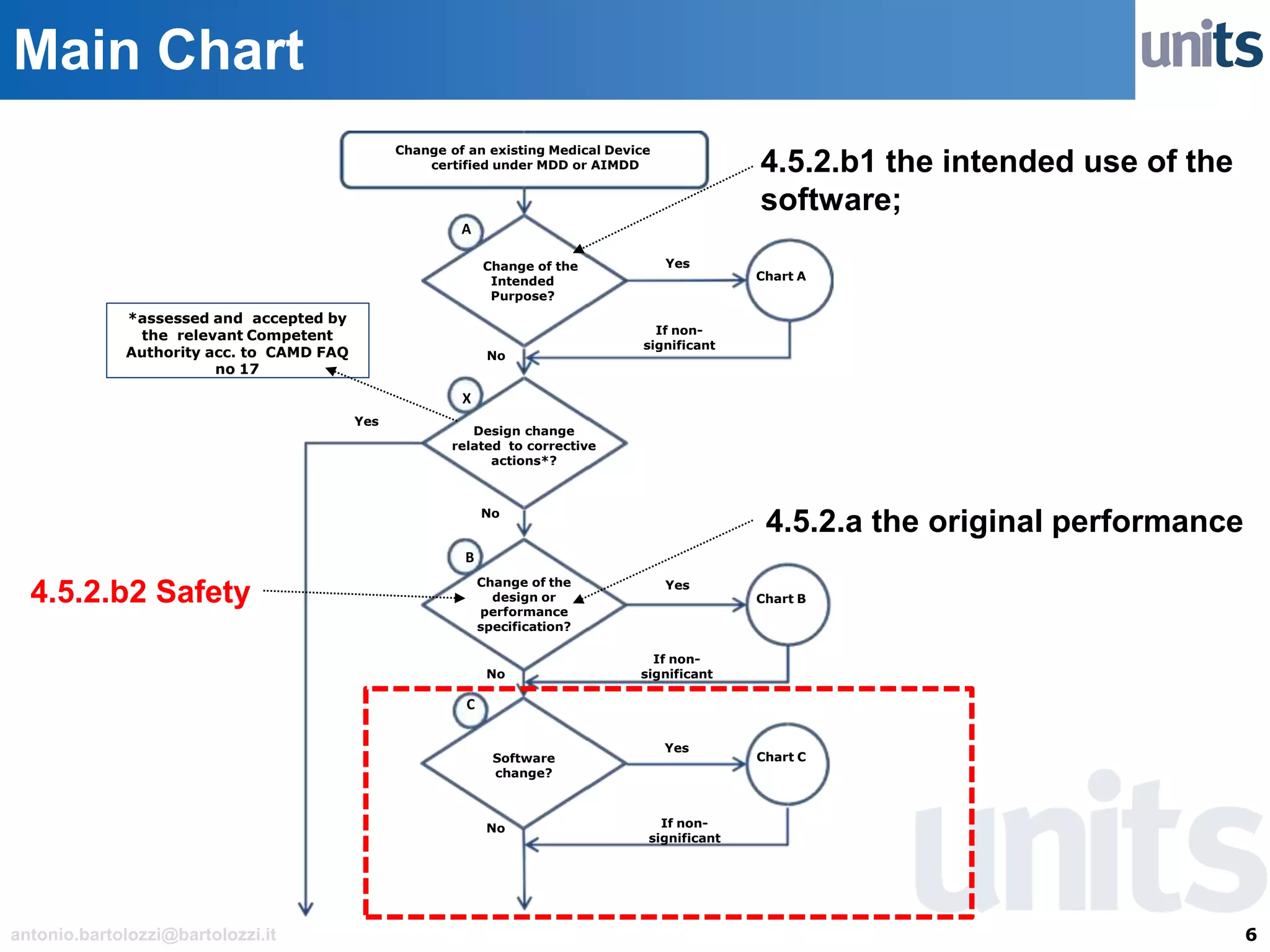
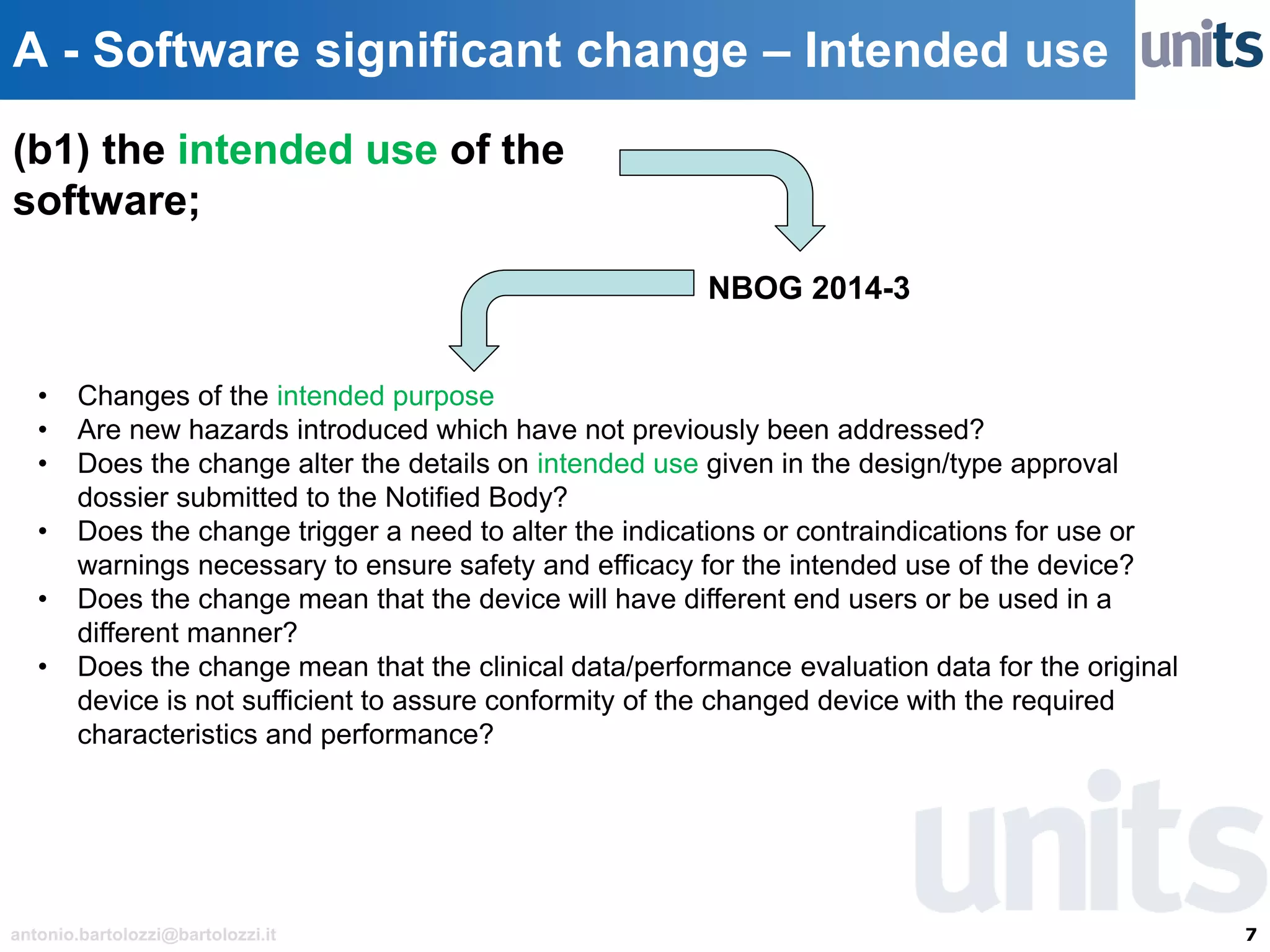
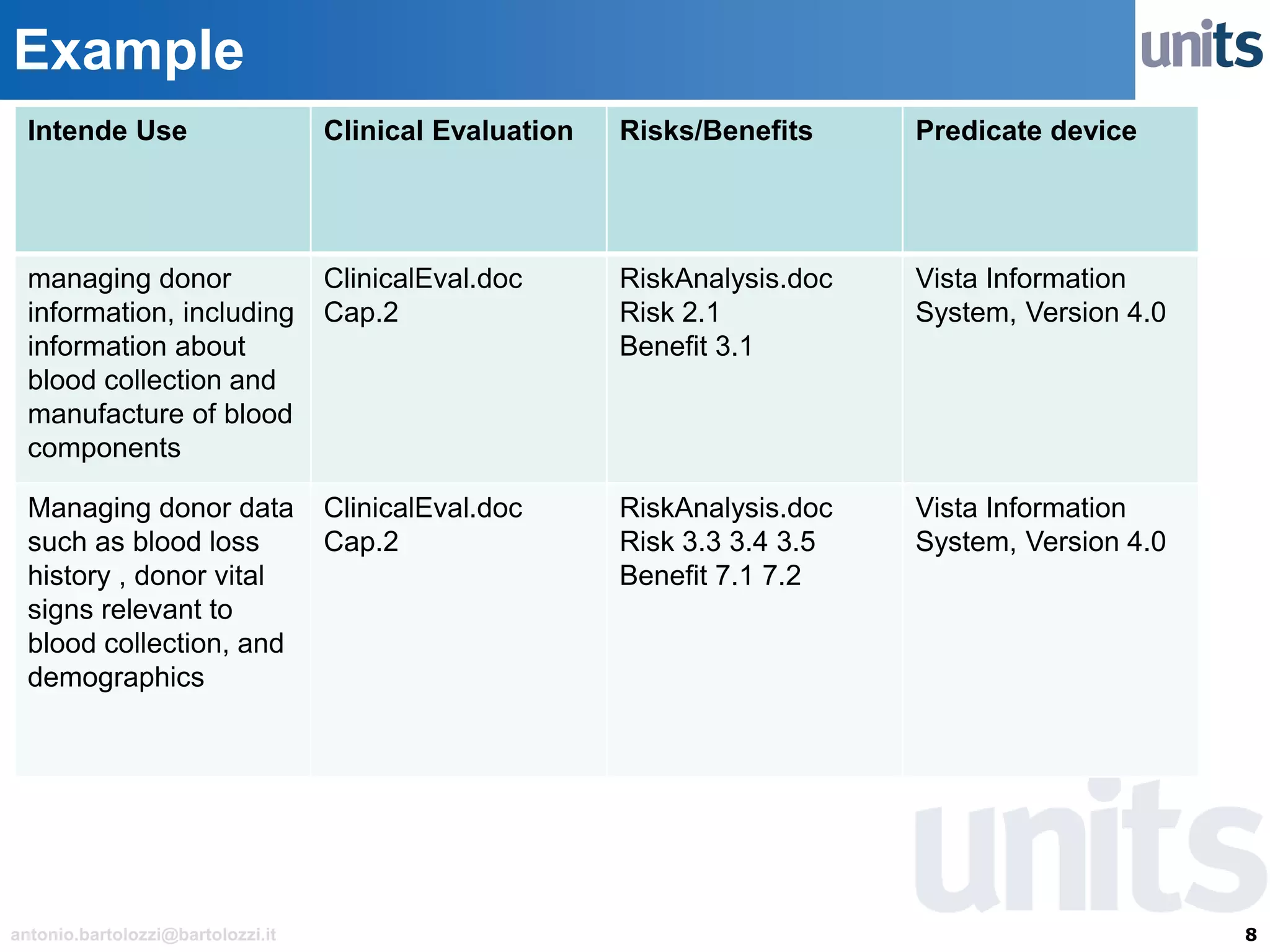
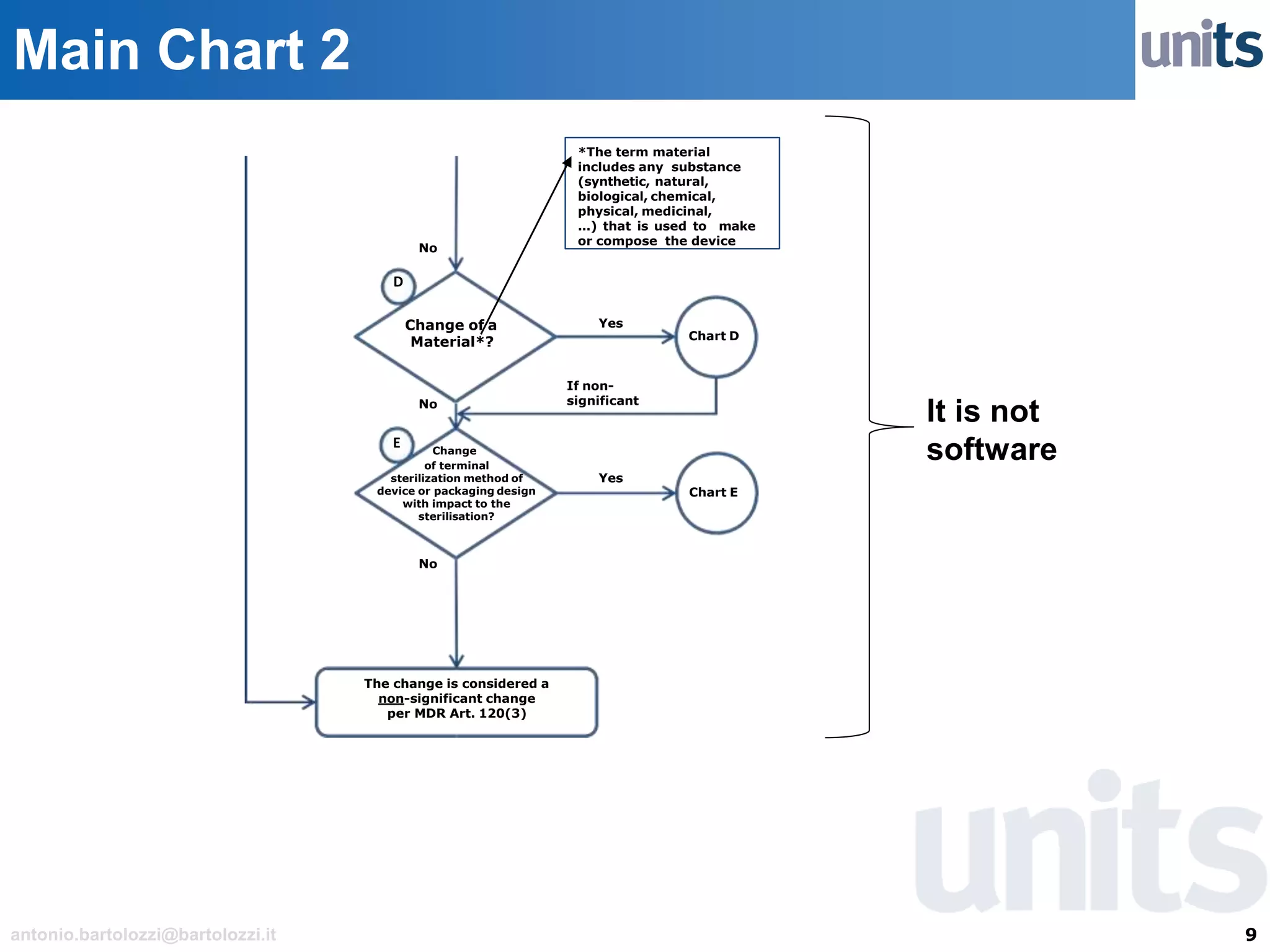
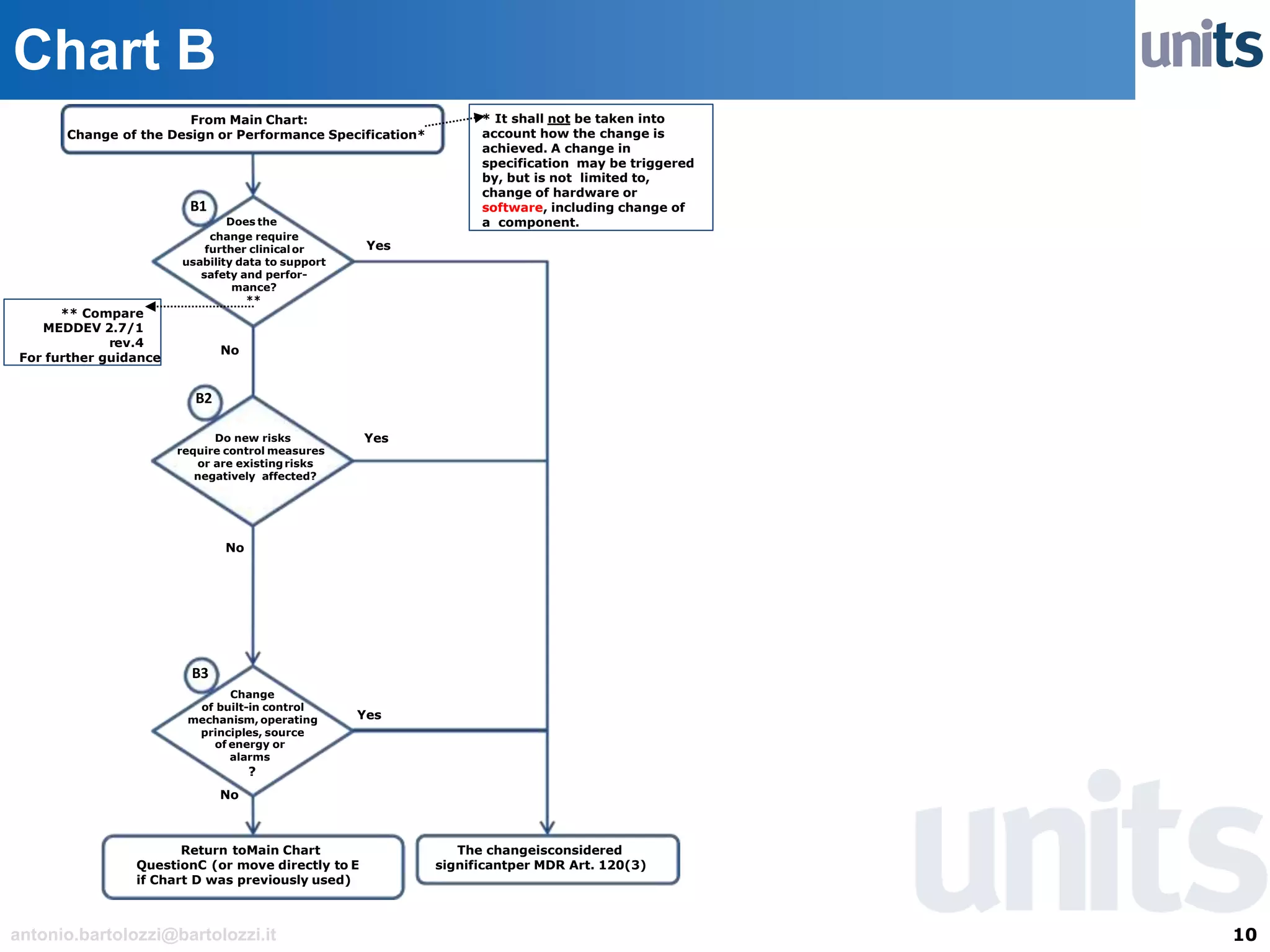
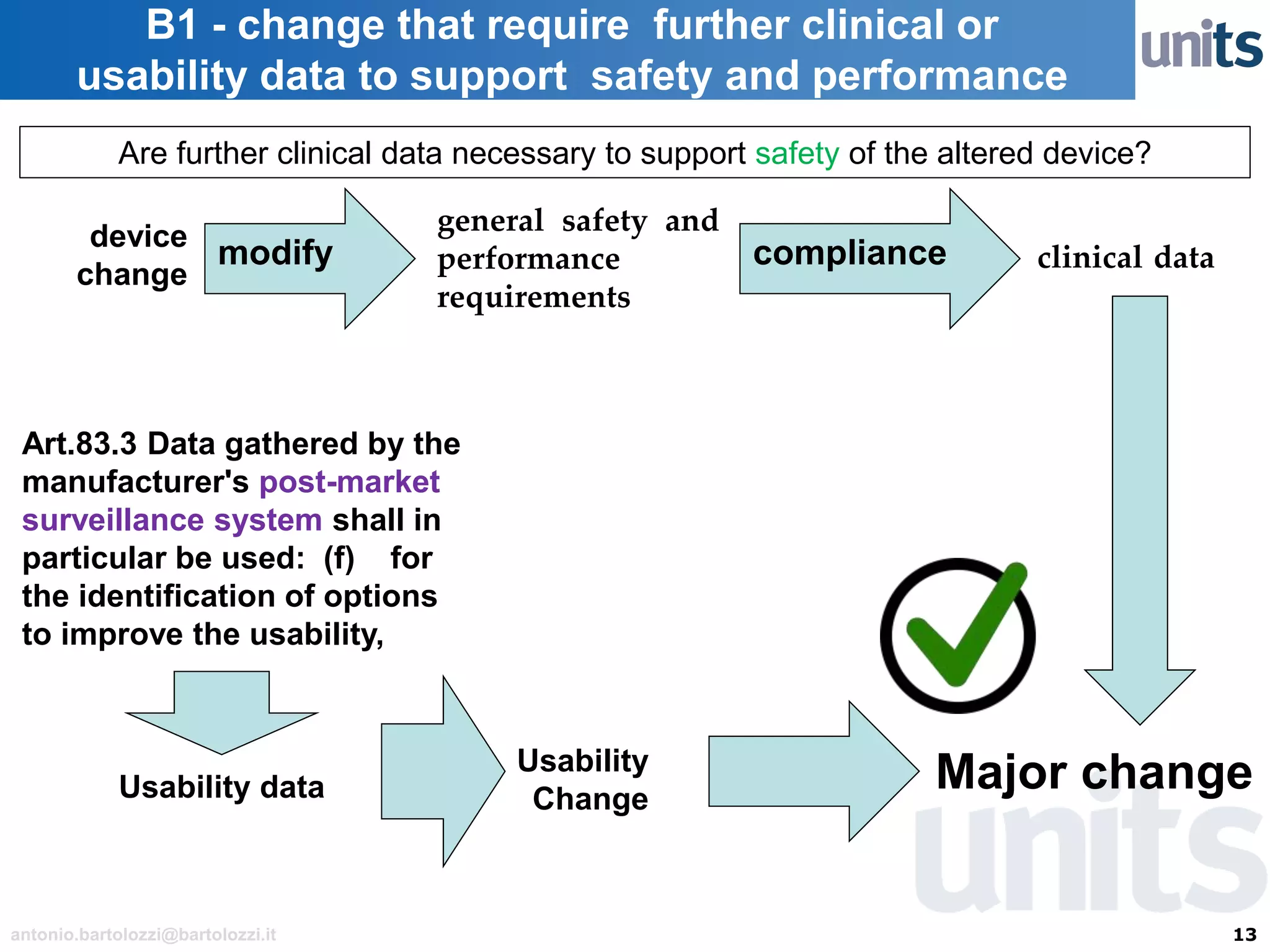
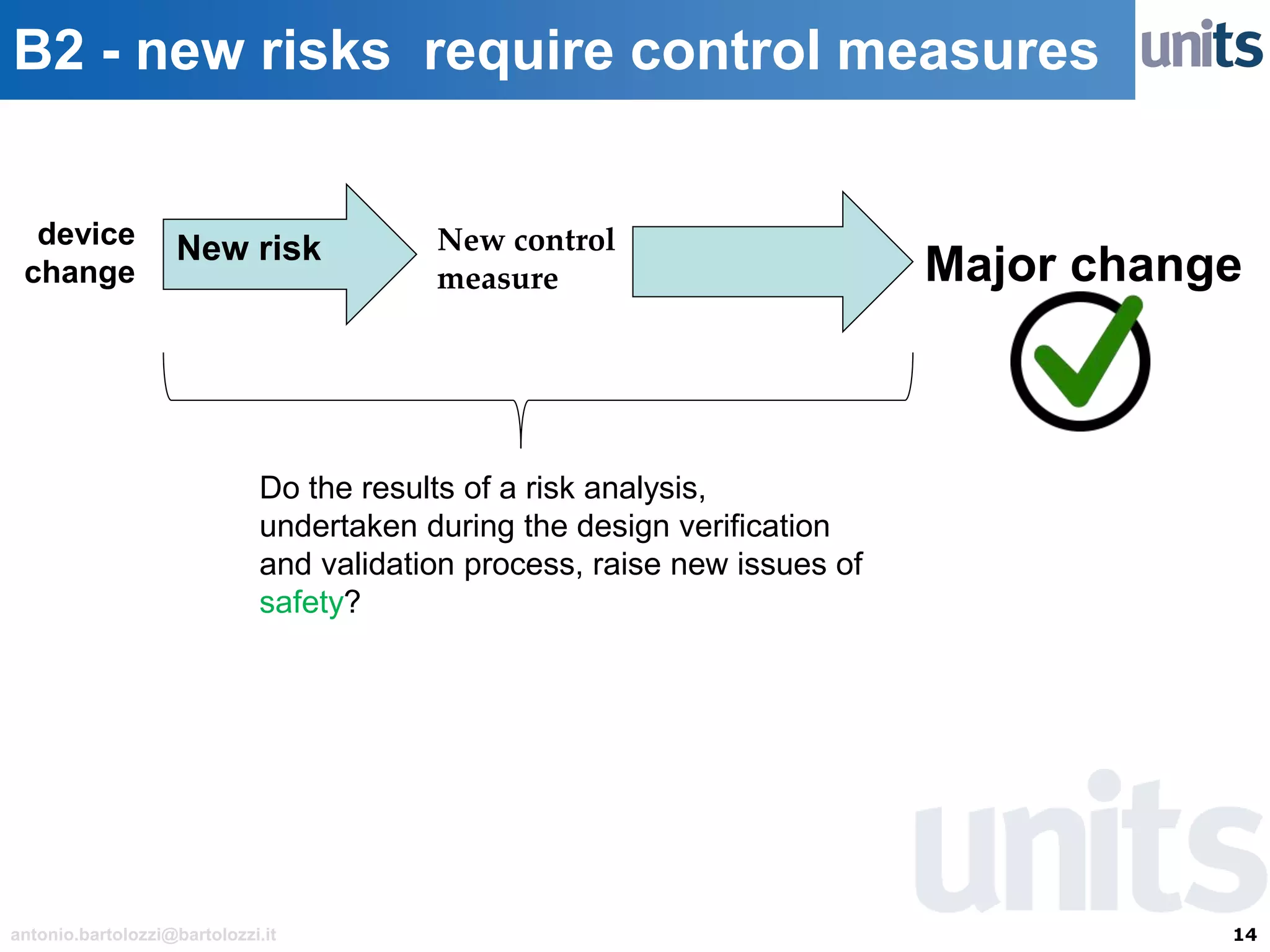
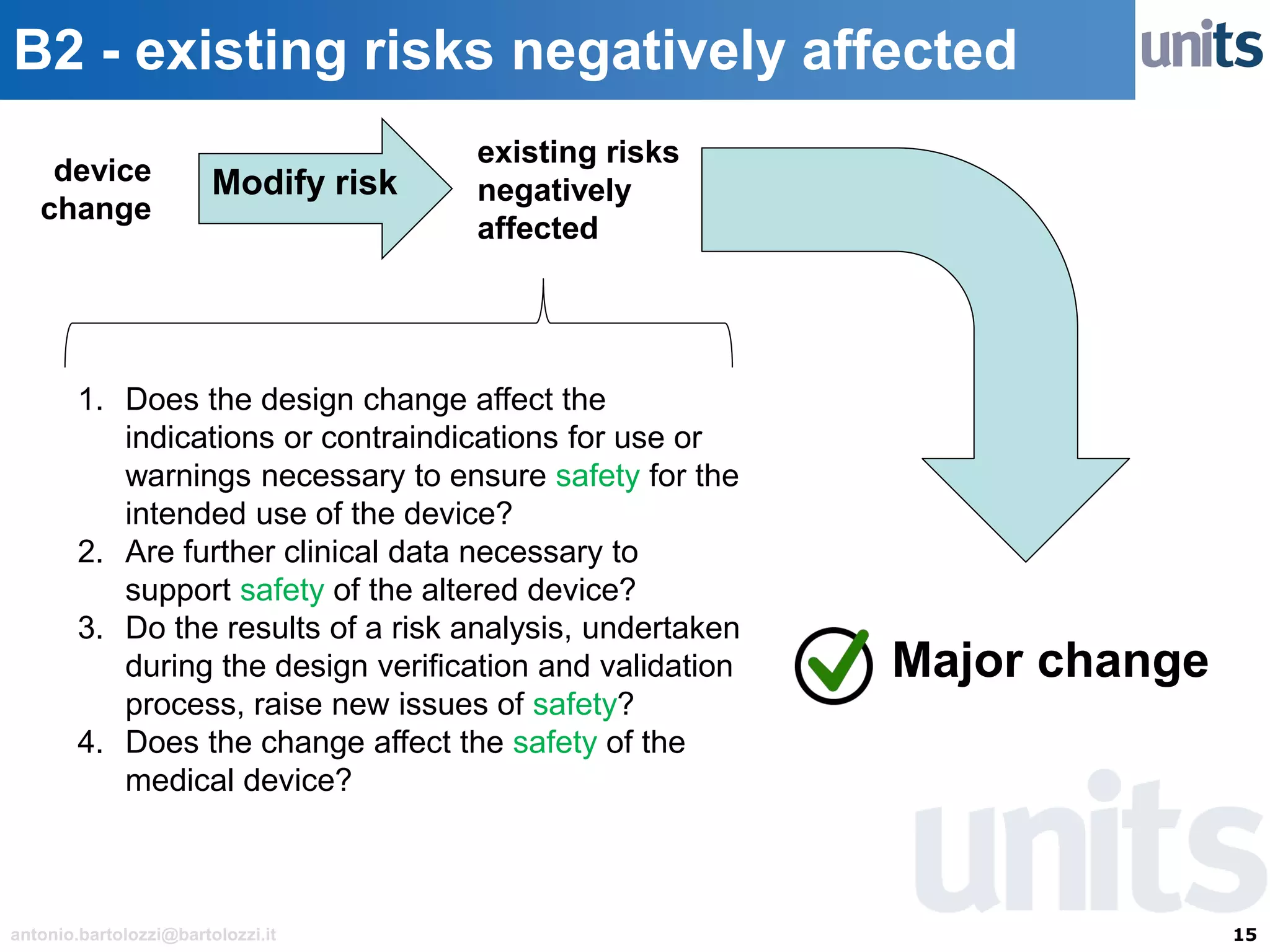
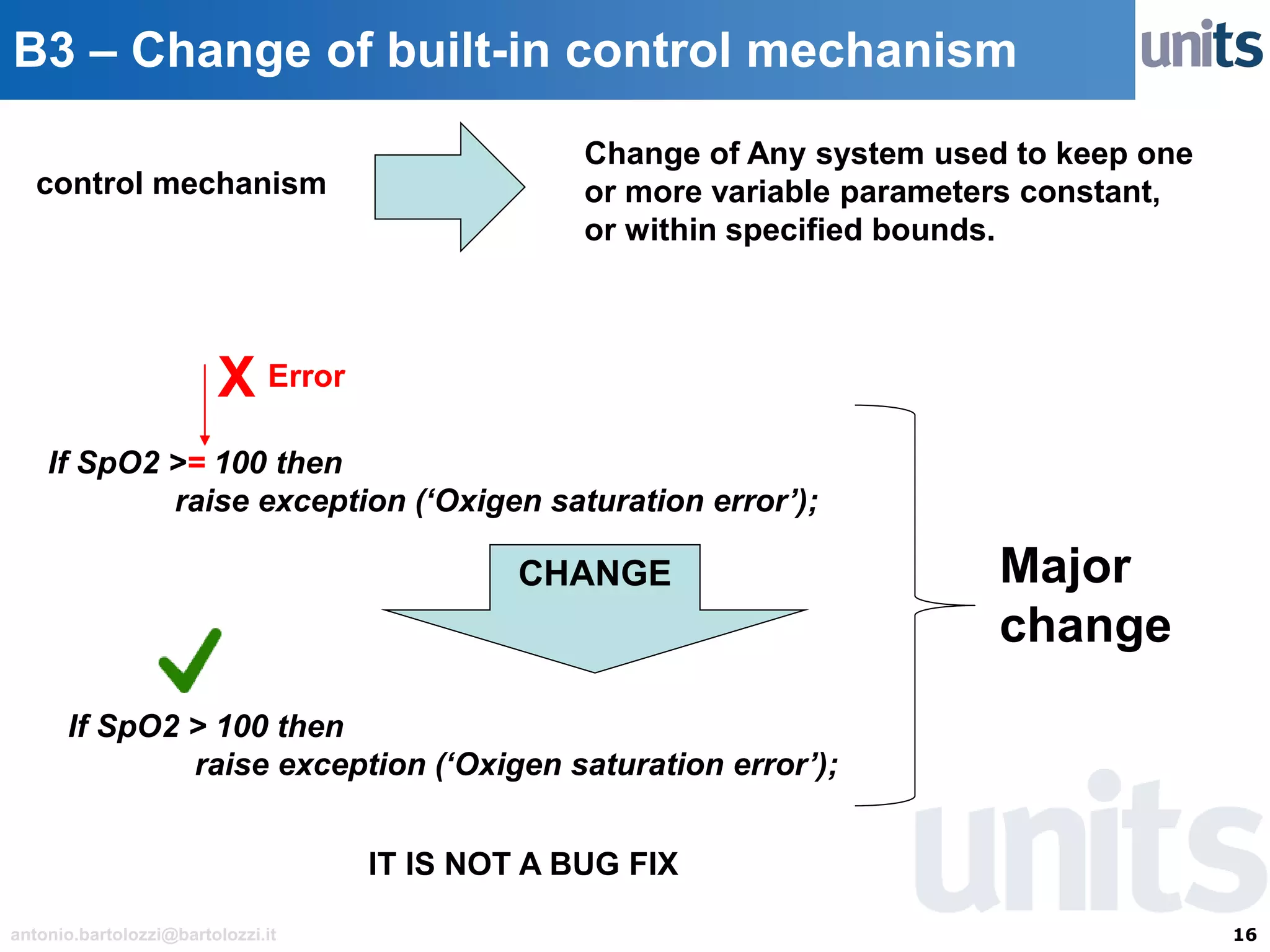
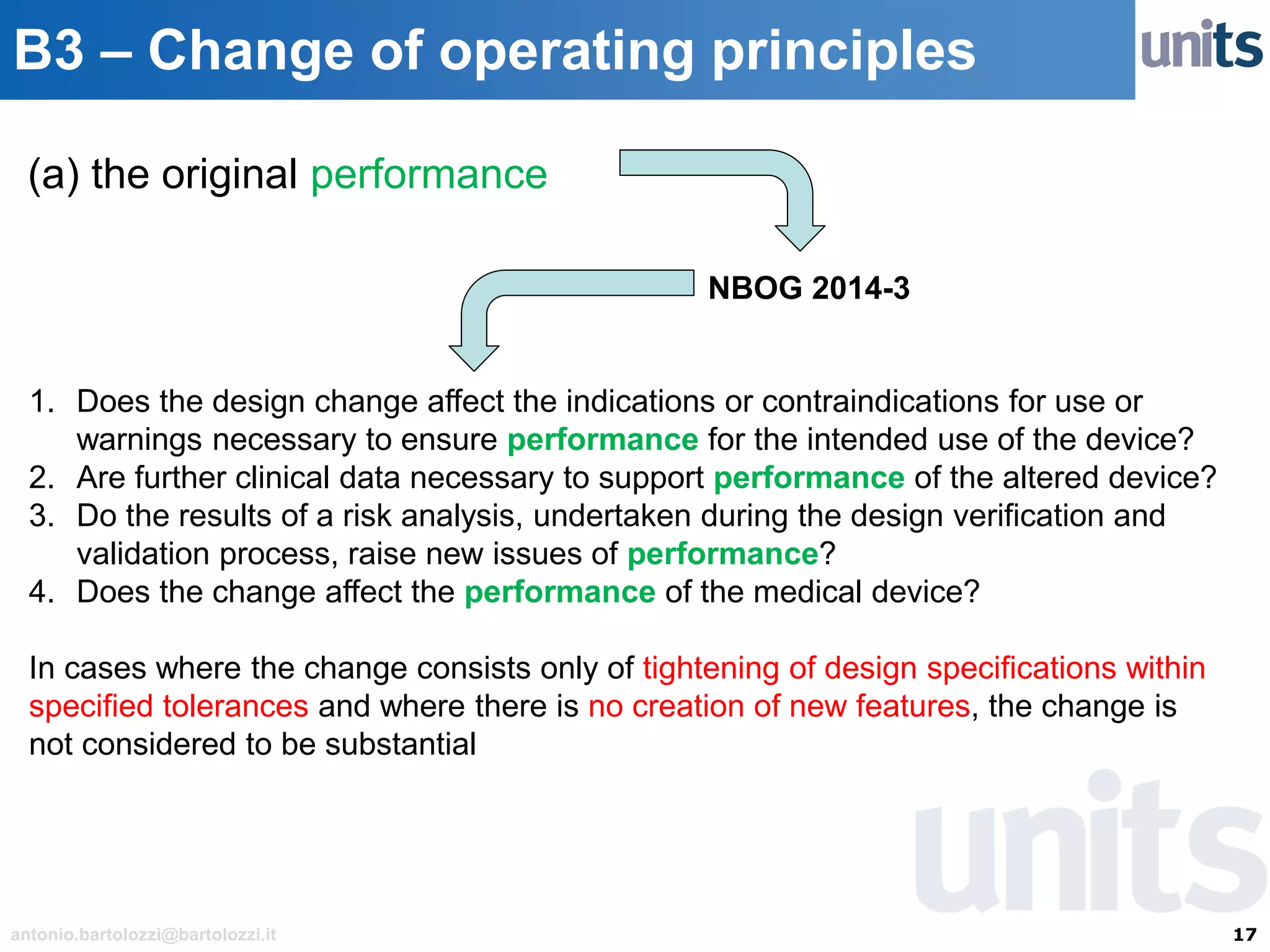
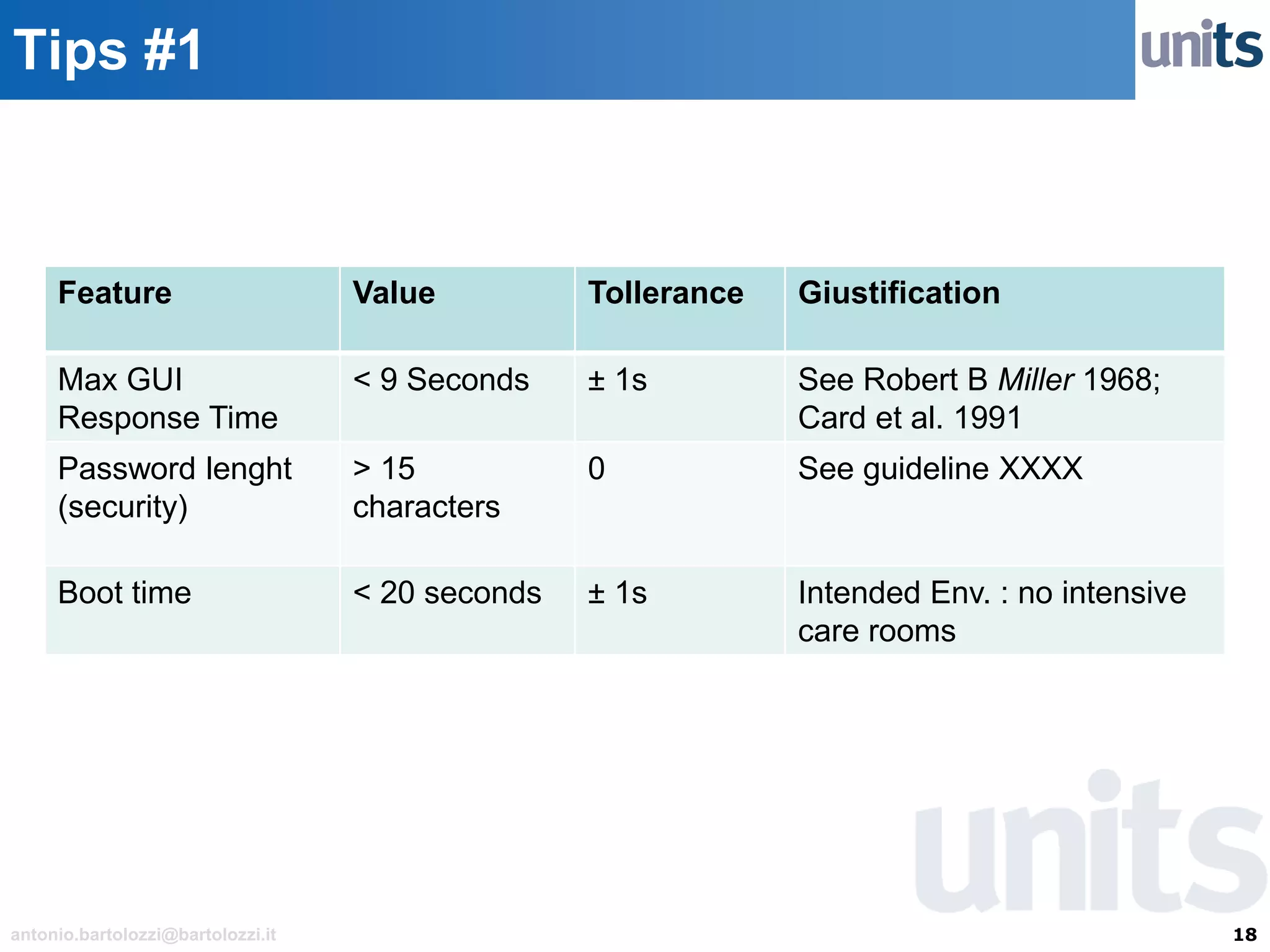
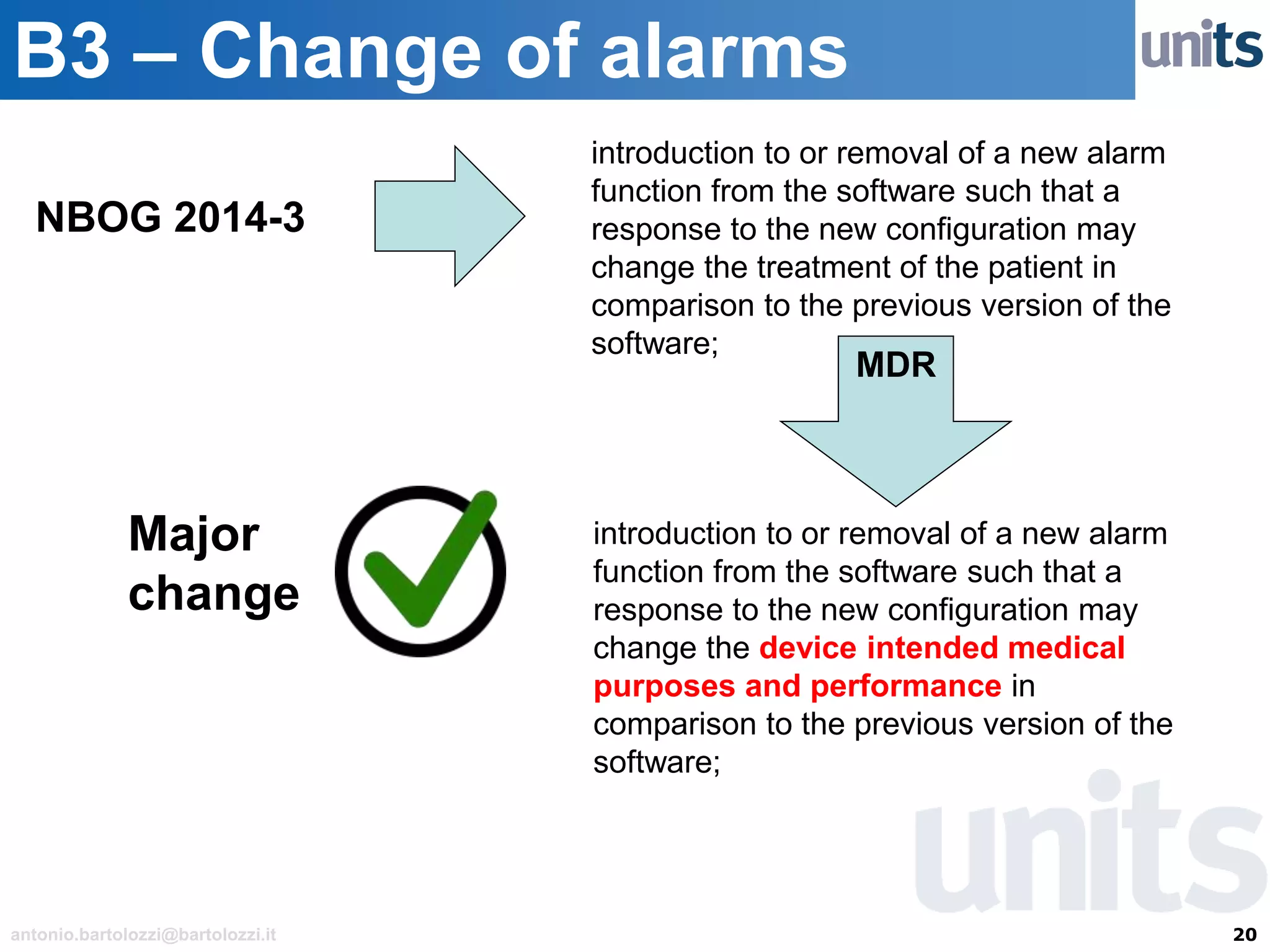
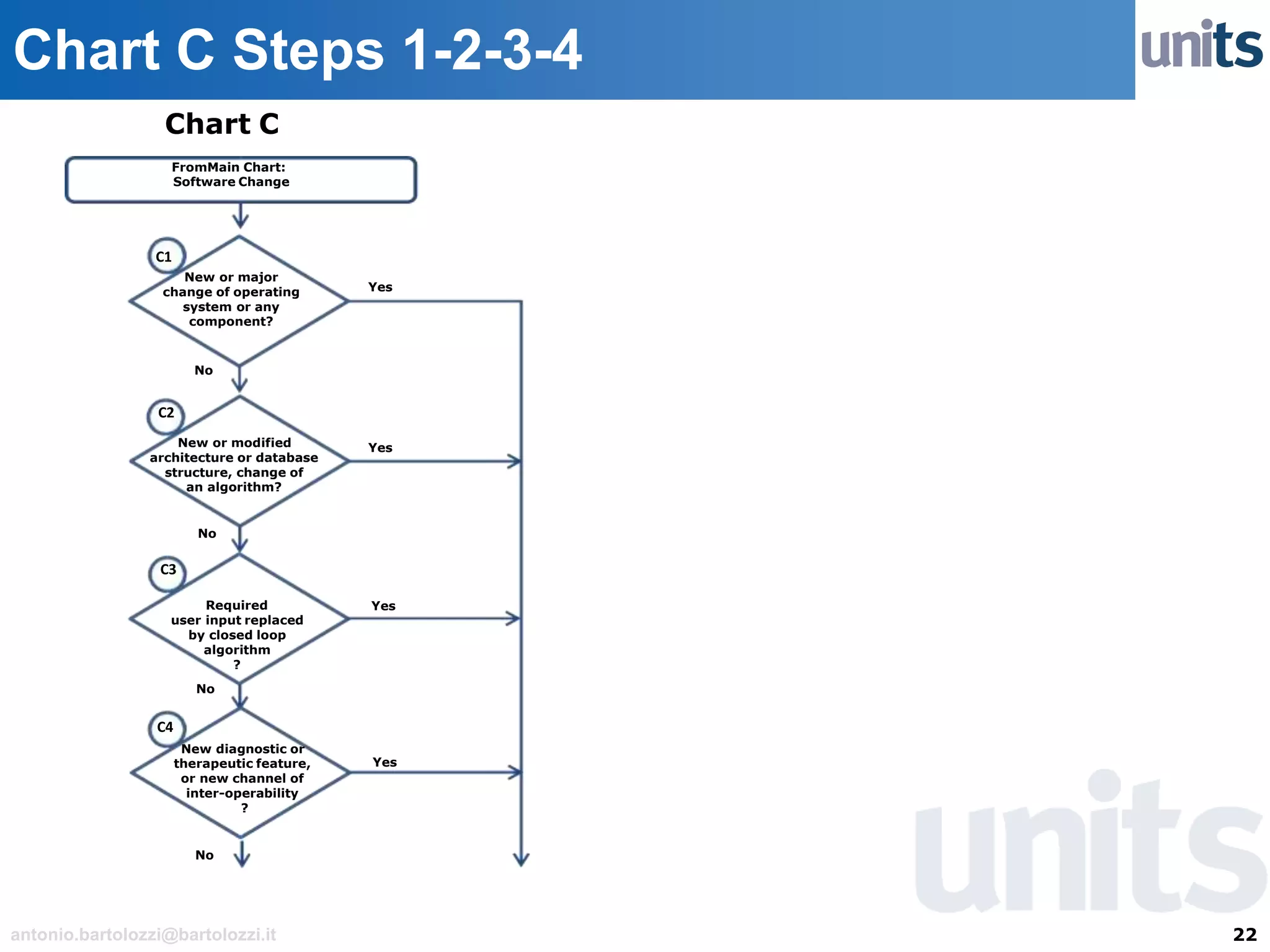
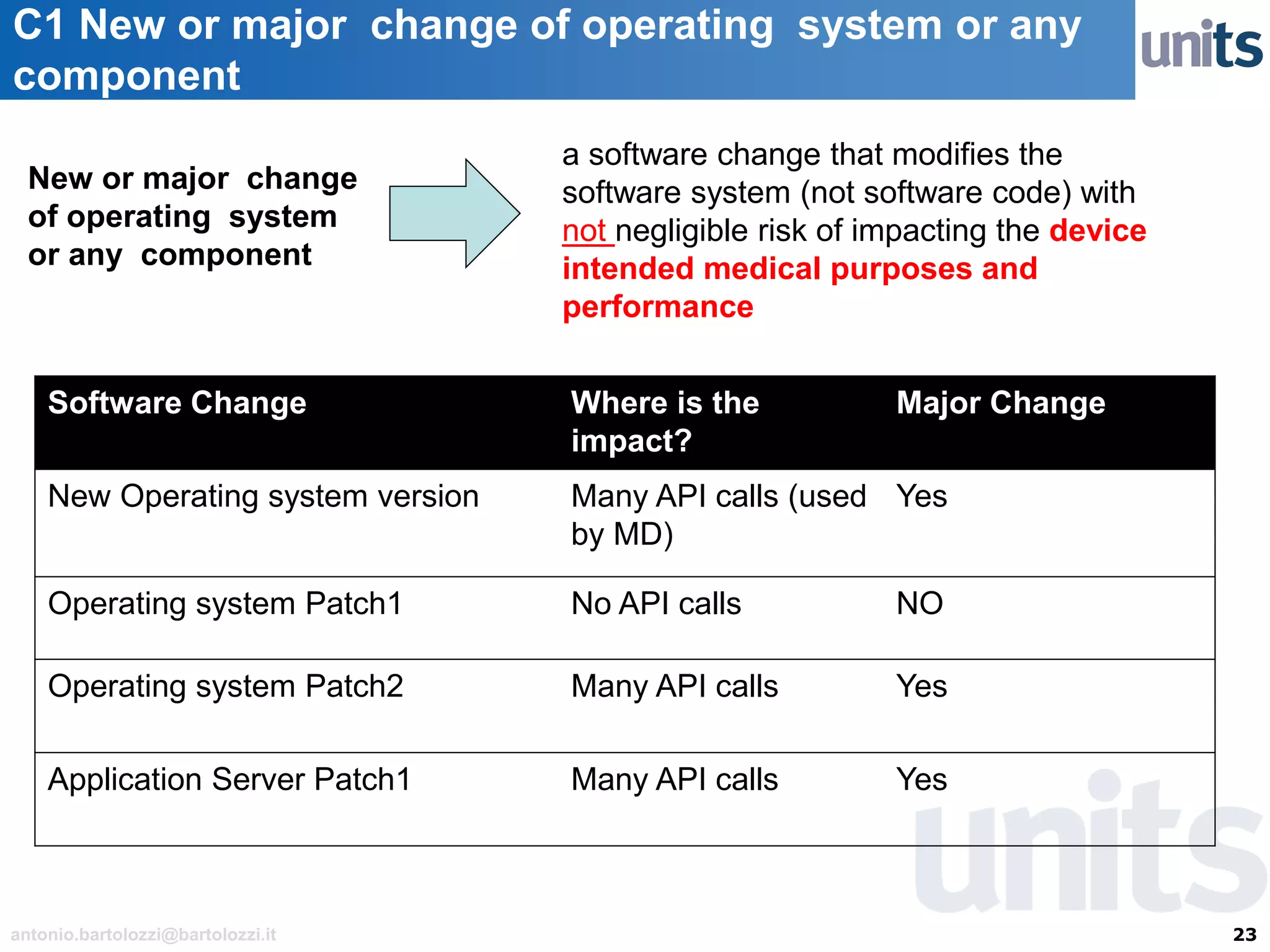
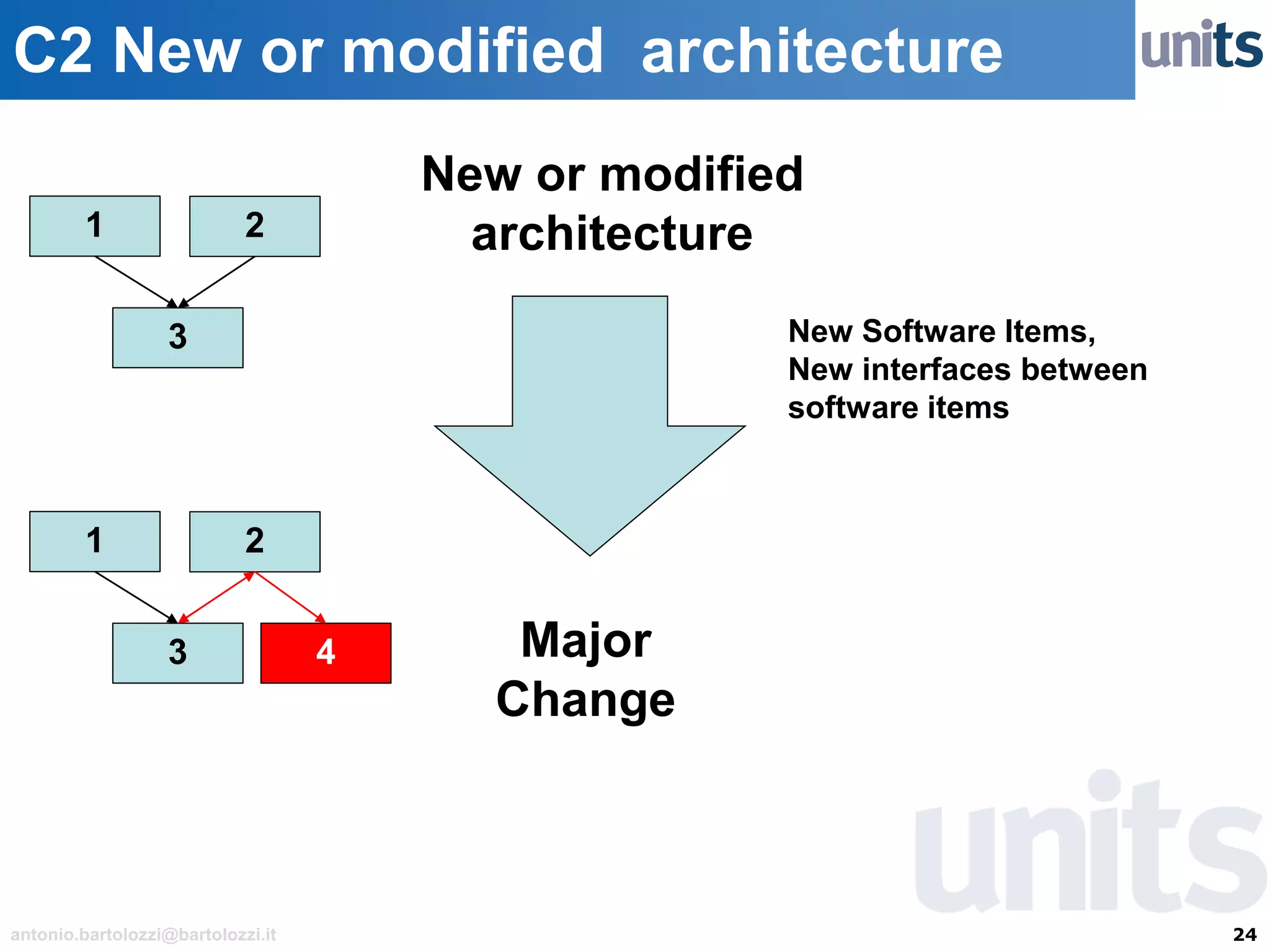
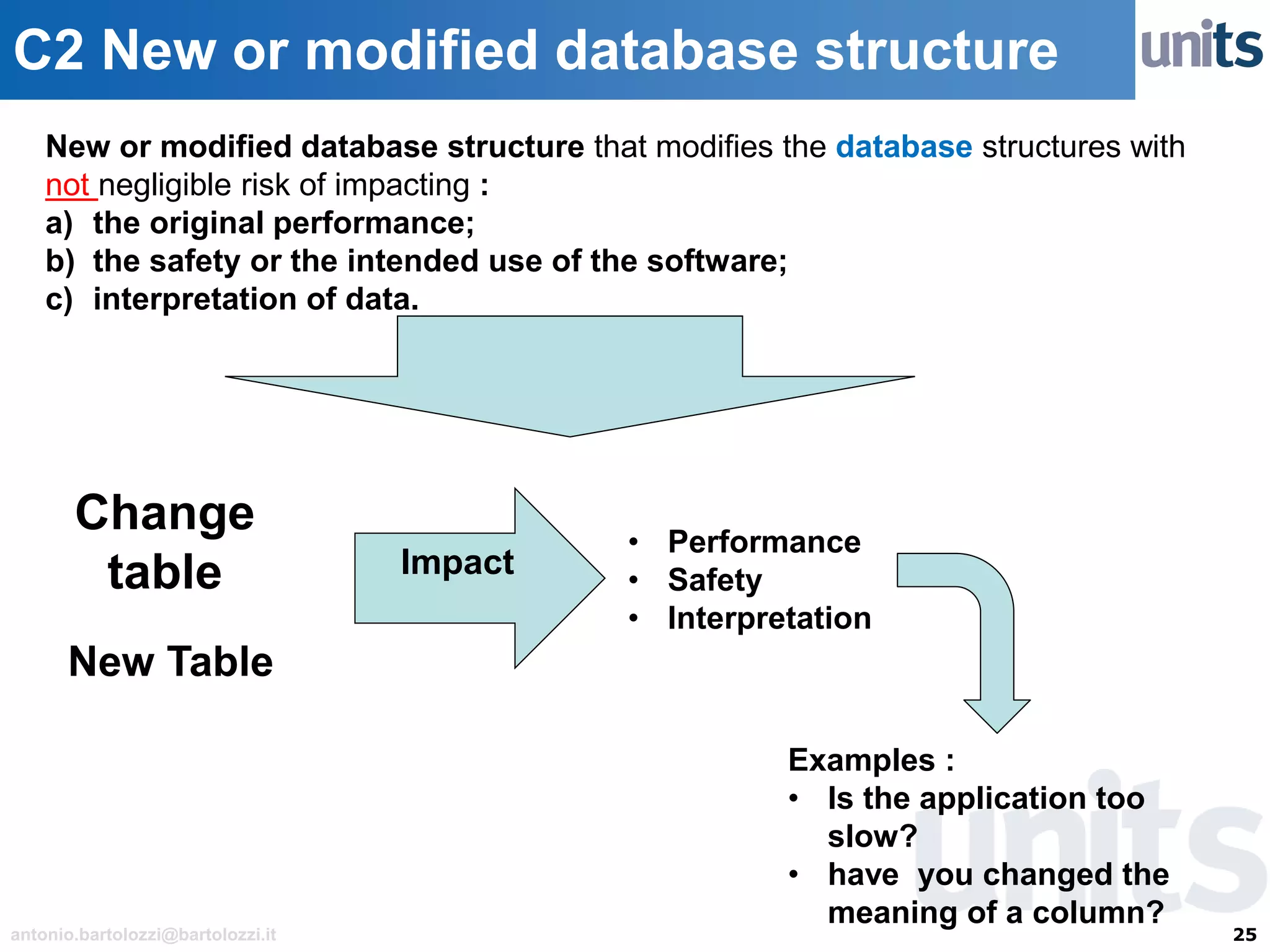
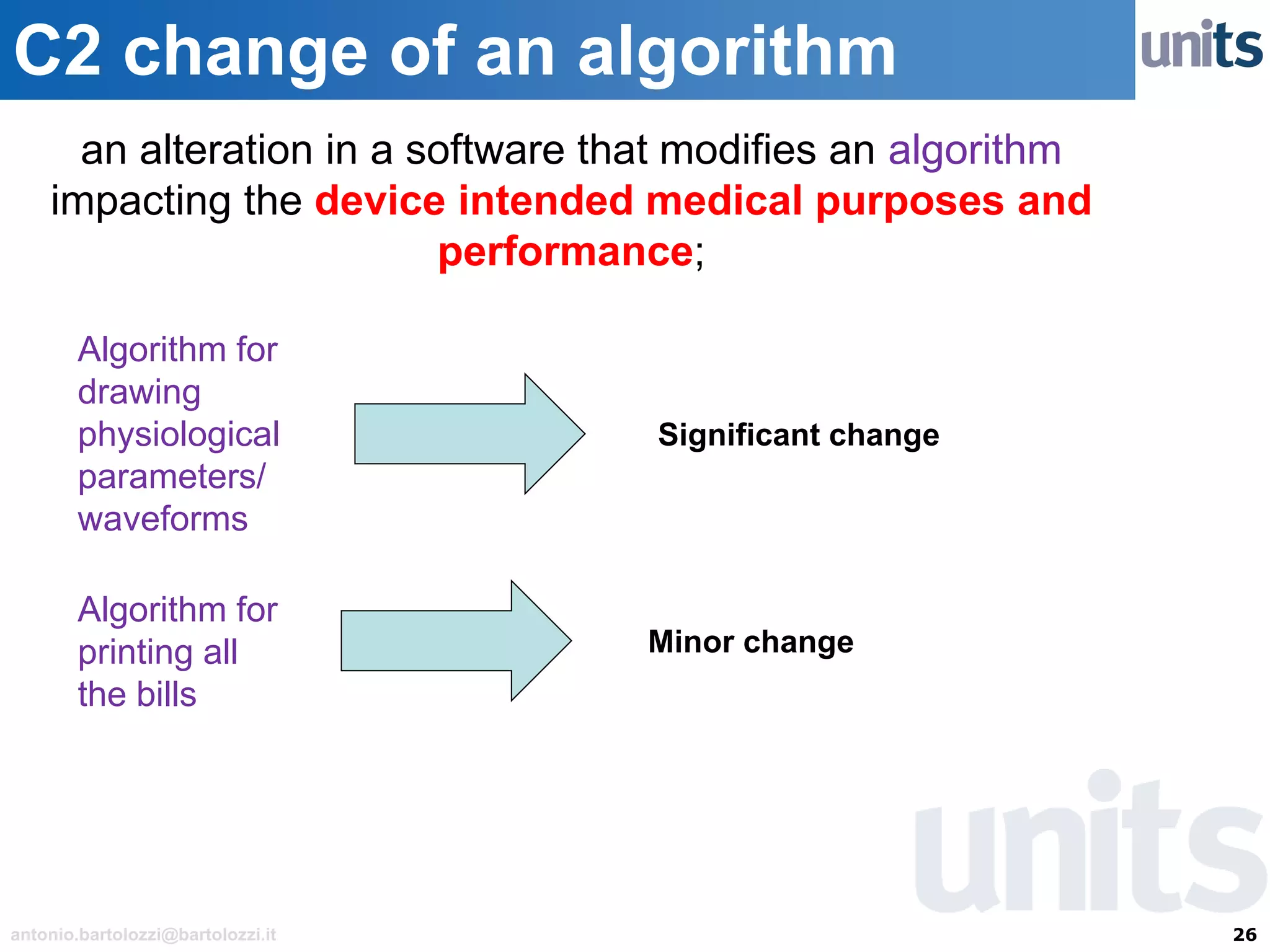
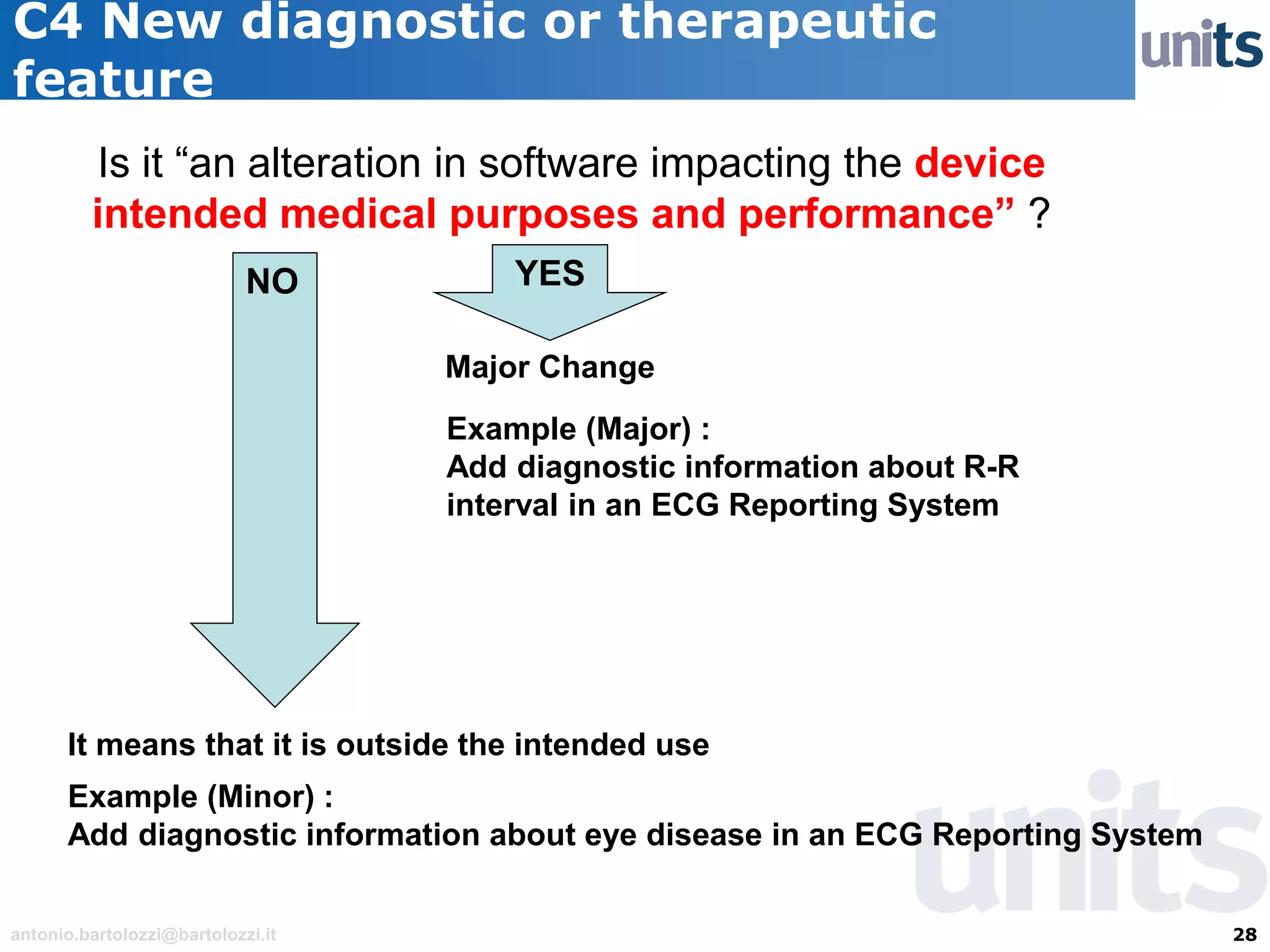
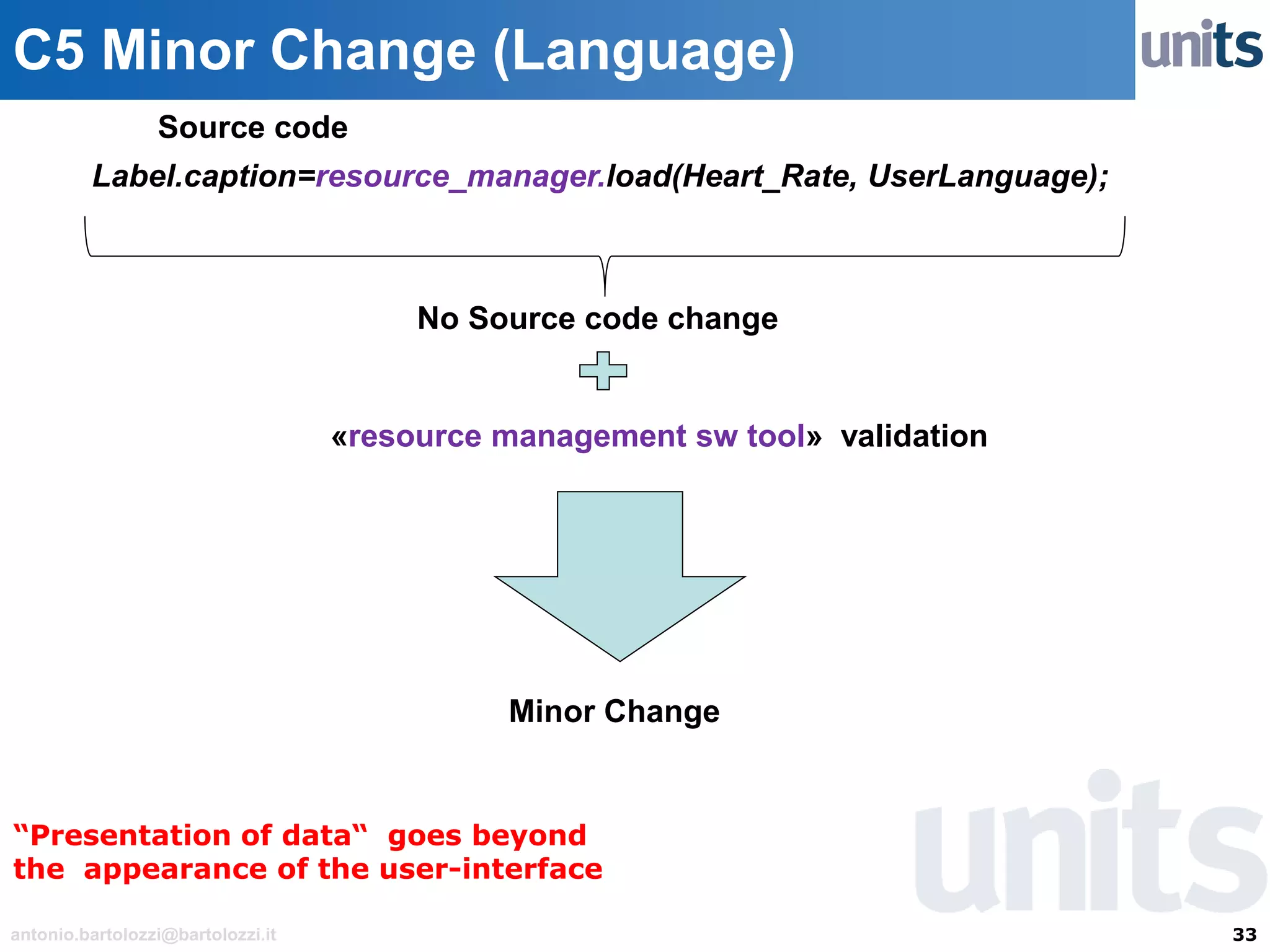
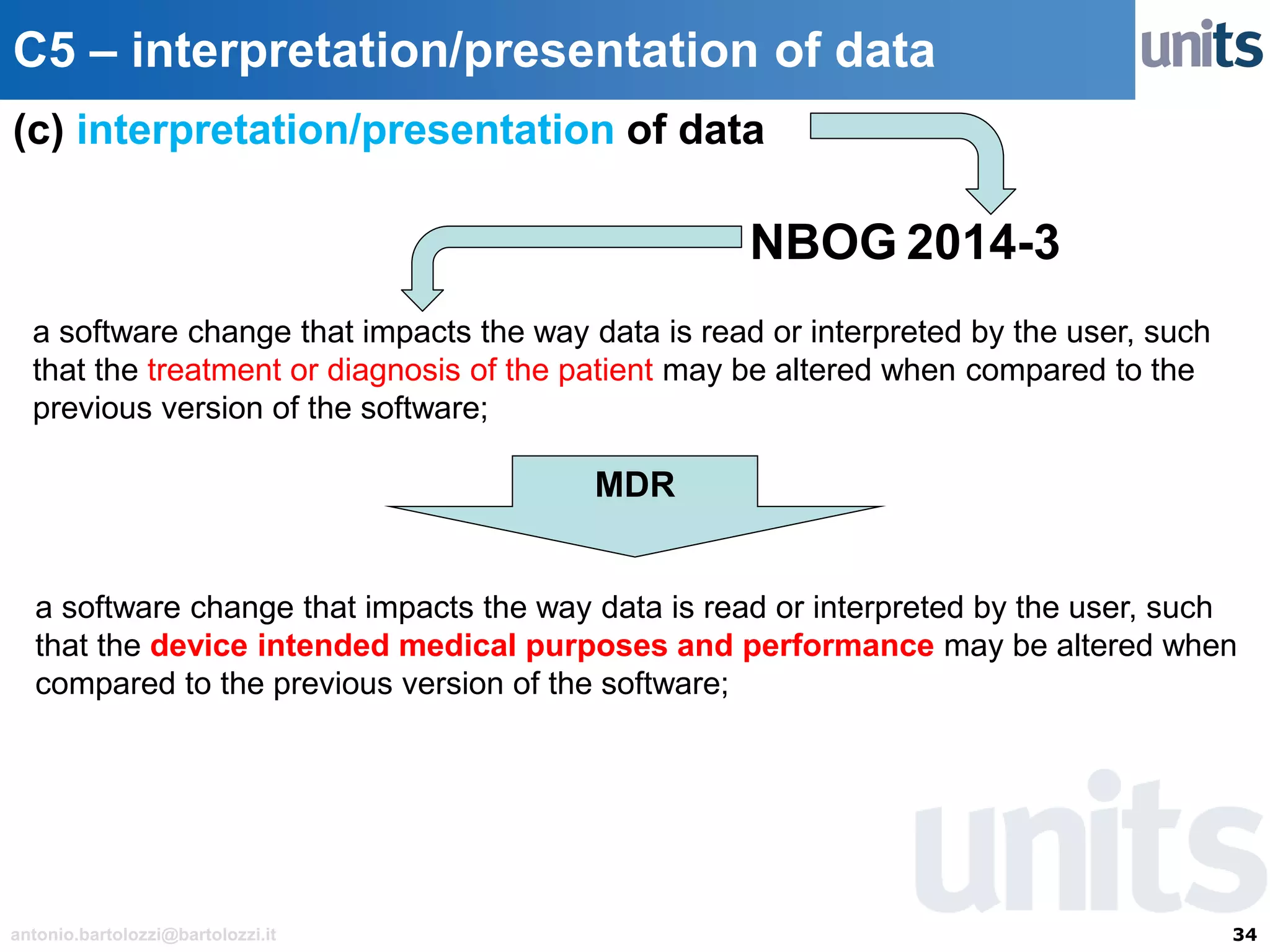
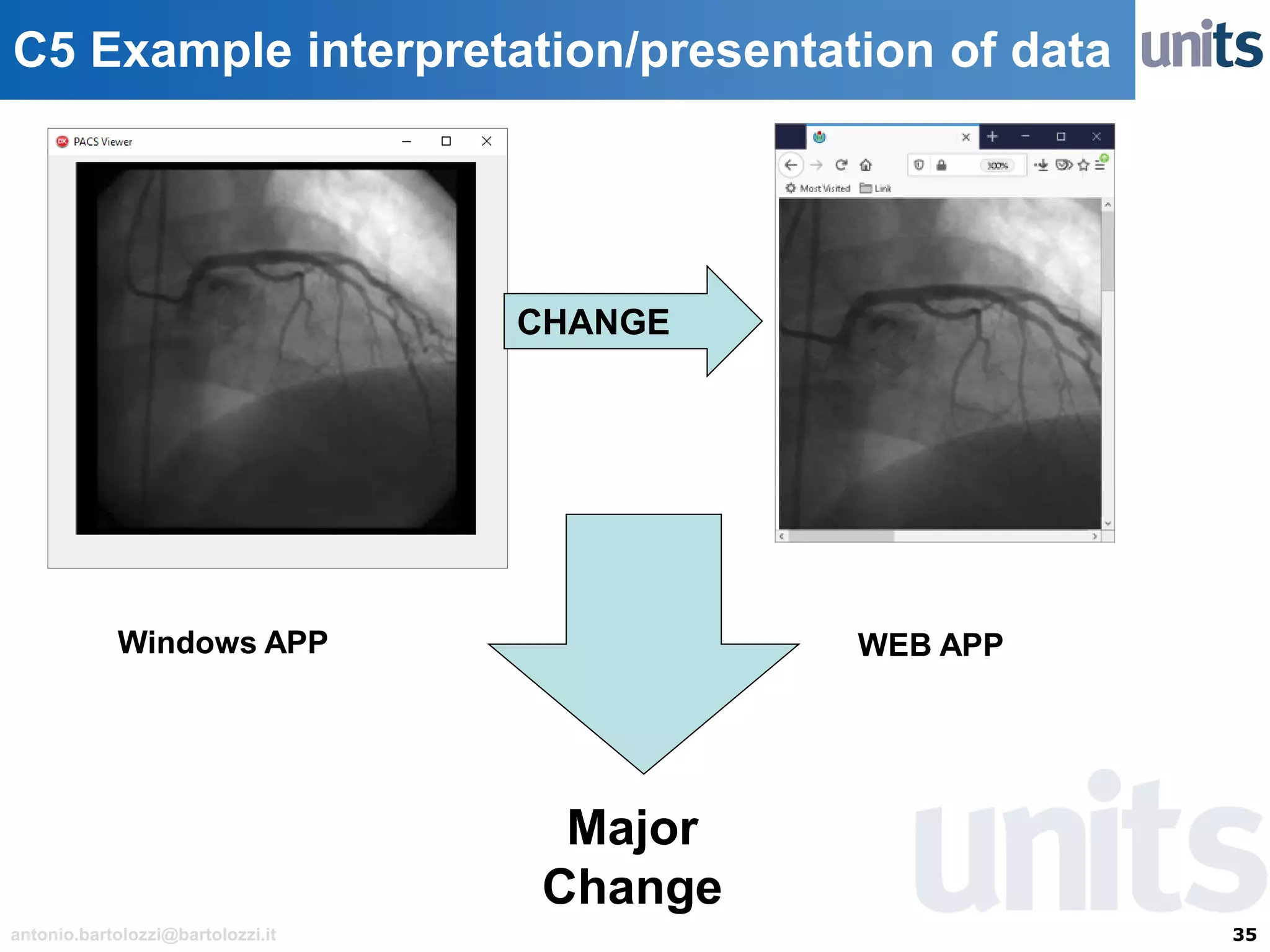
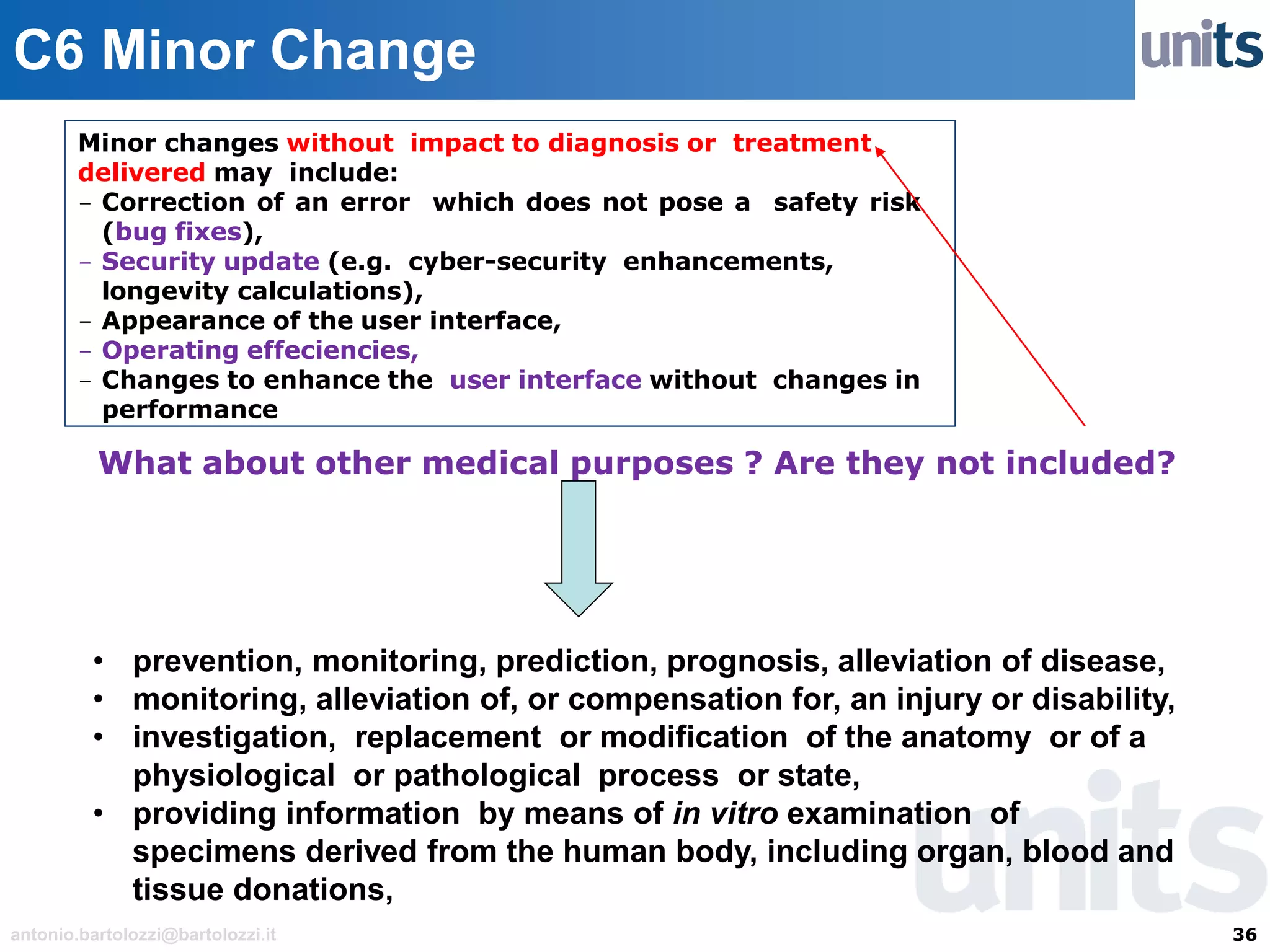
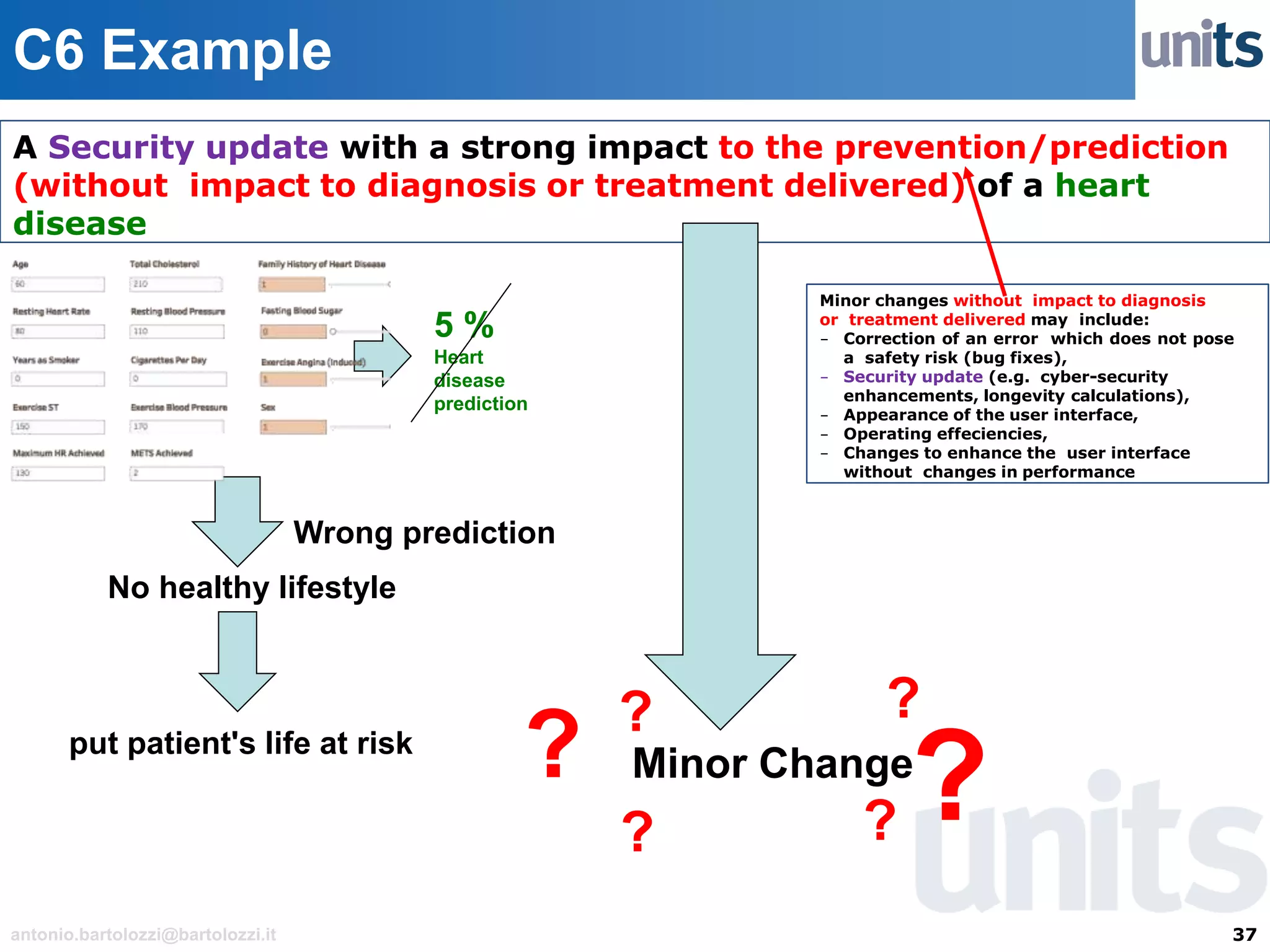
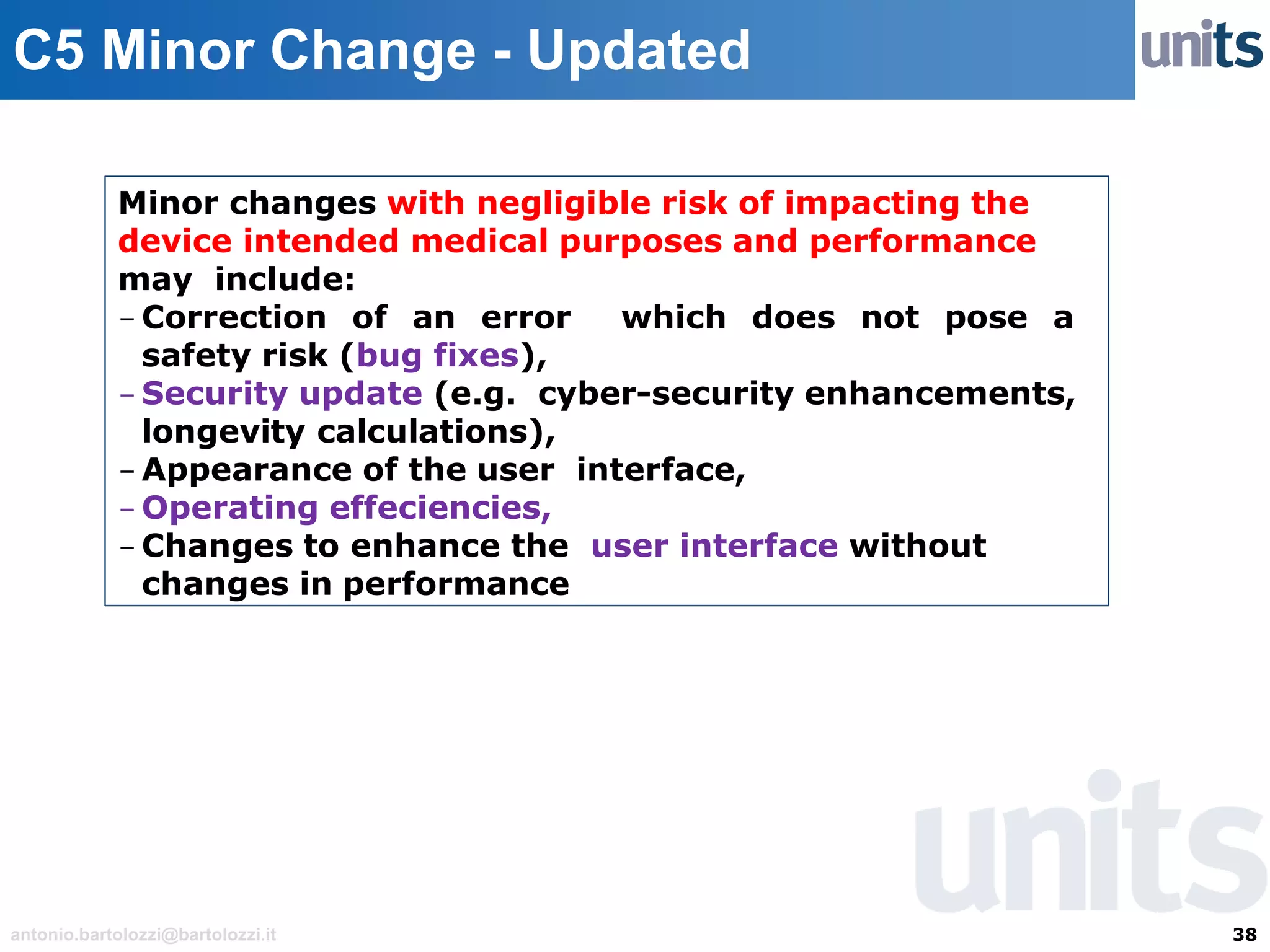
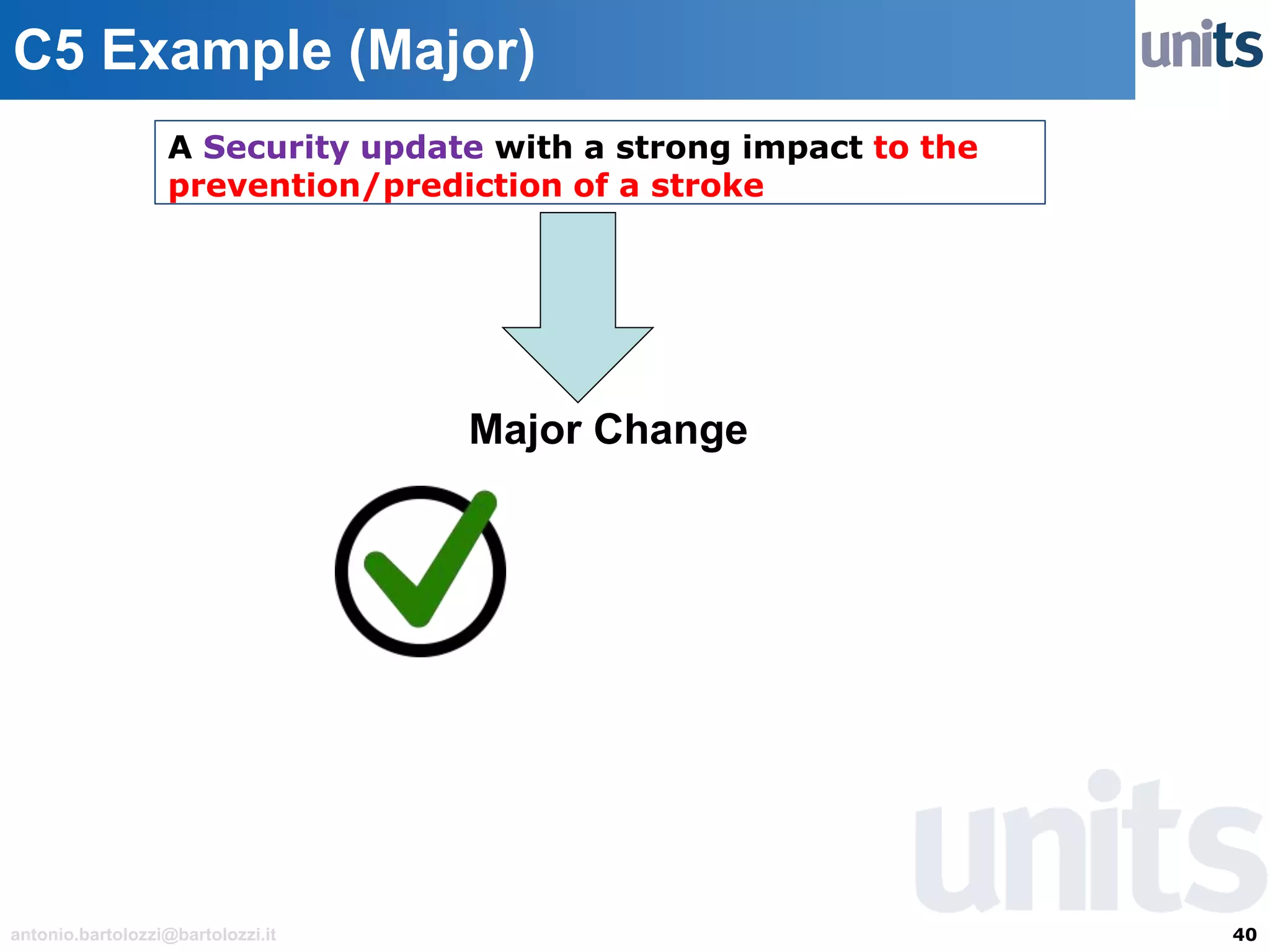
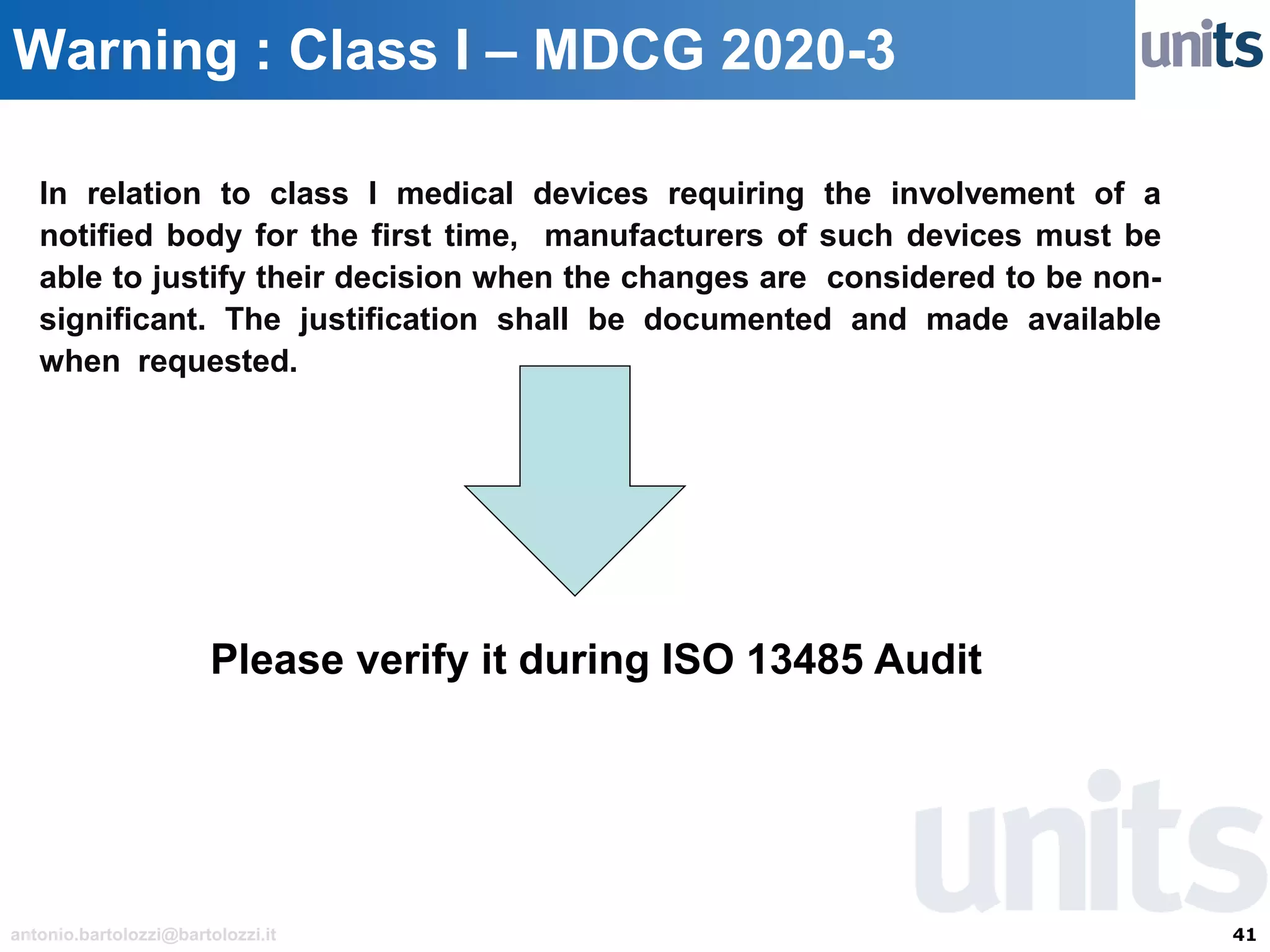
The document provides guidance on significant changes related to medical devices and software under the MDR. It outlines criteria for determining when changes, such as modifications to design, algorithms, or intended use, necessitate new Unique Device Identifiers (UDI). Additionally, it discusses the implications of minor software revisions, highlighting the importance of safety and performance compliance through clinical data.