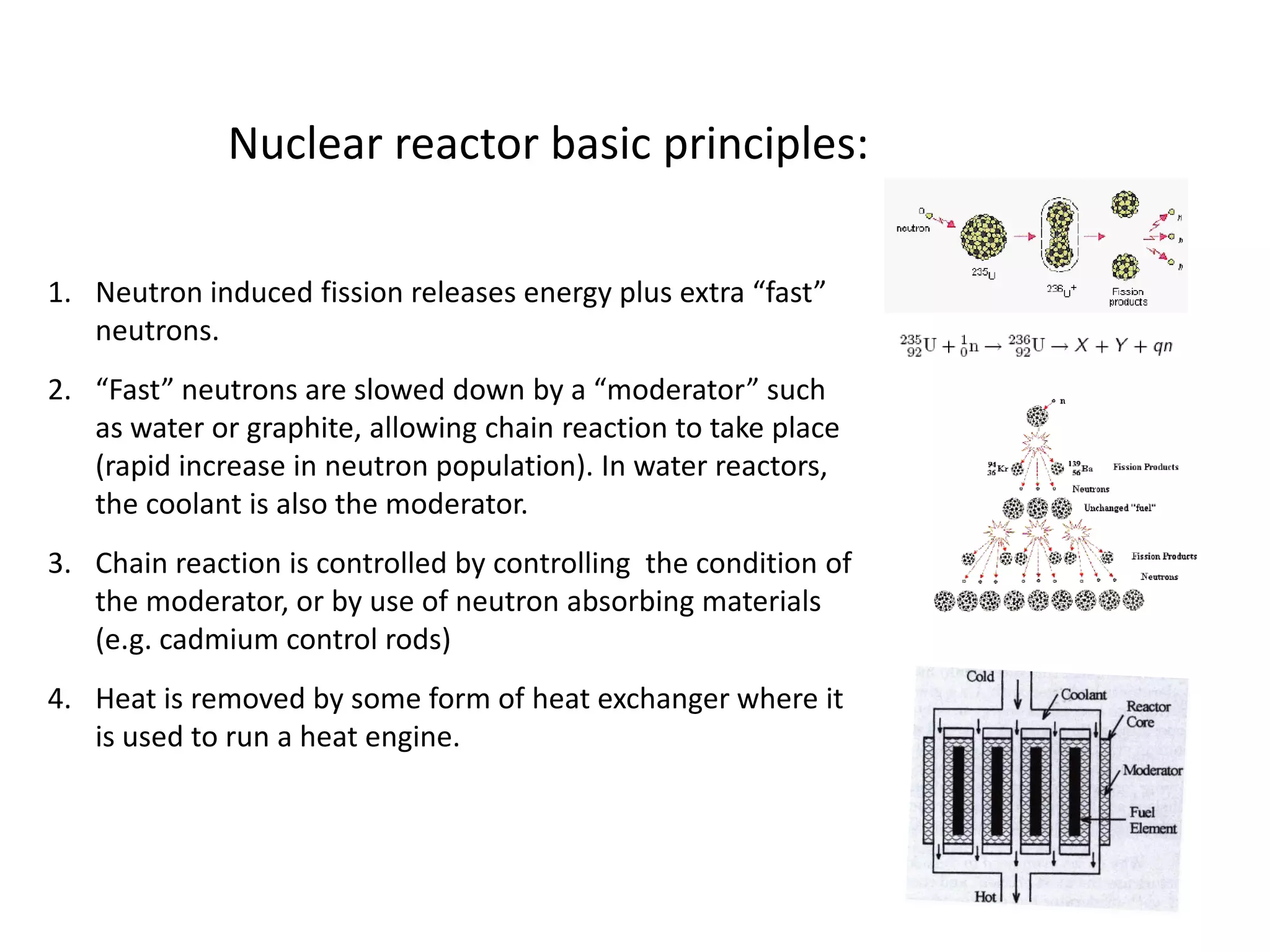
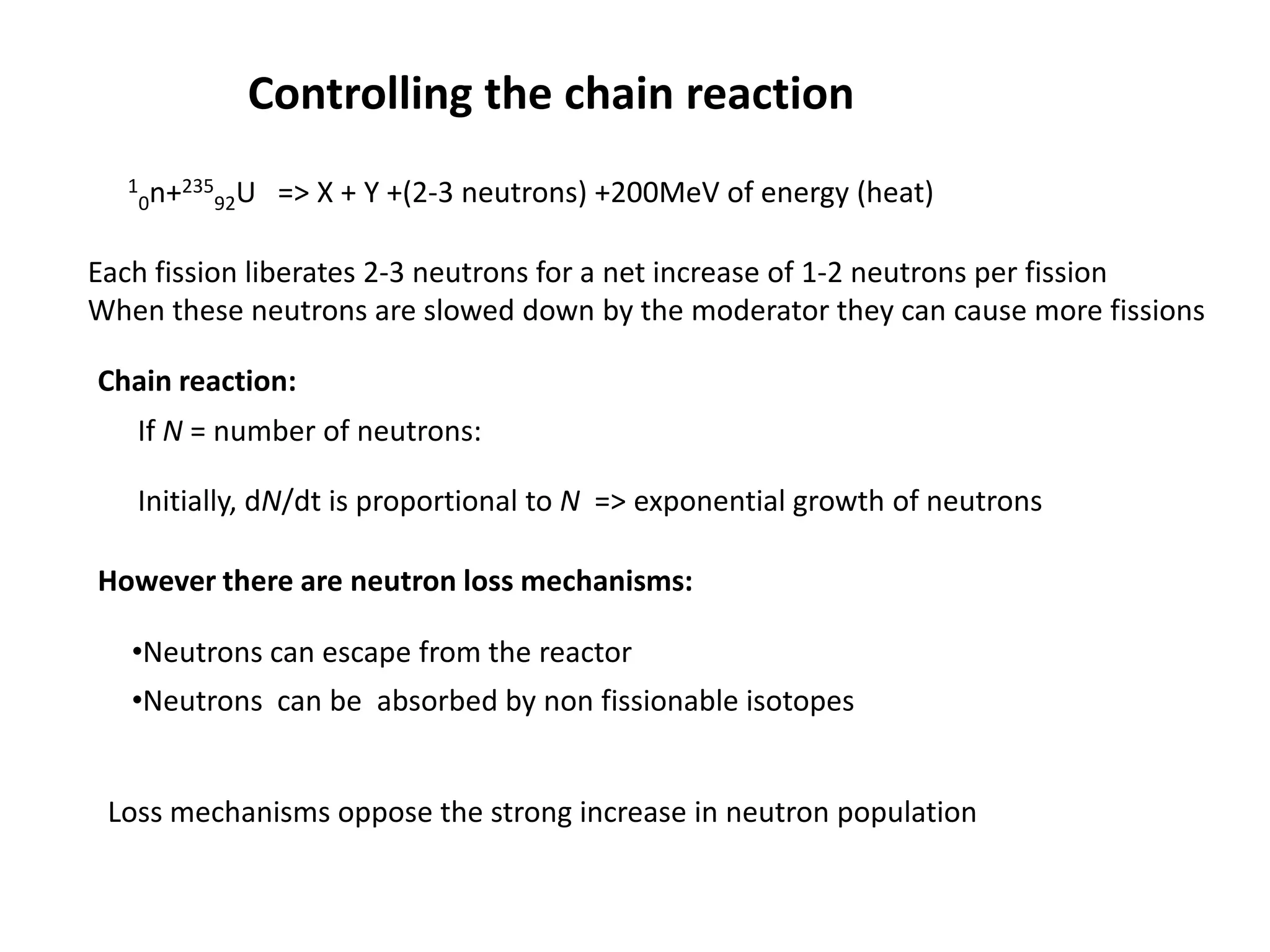
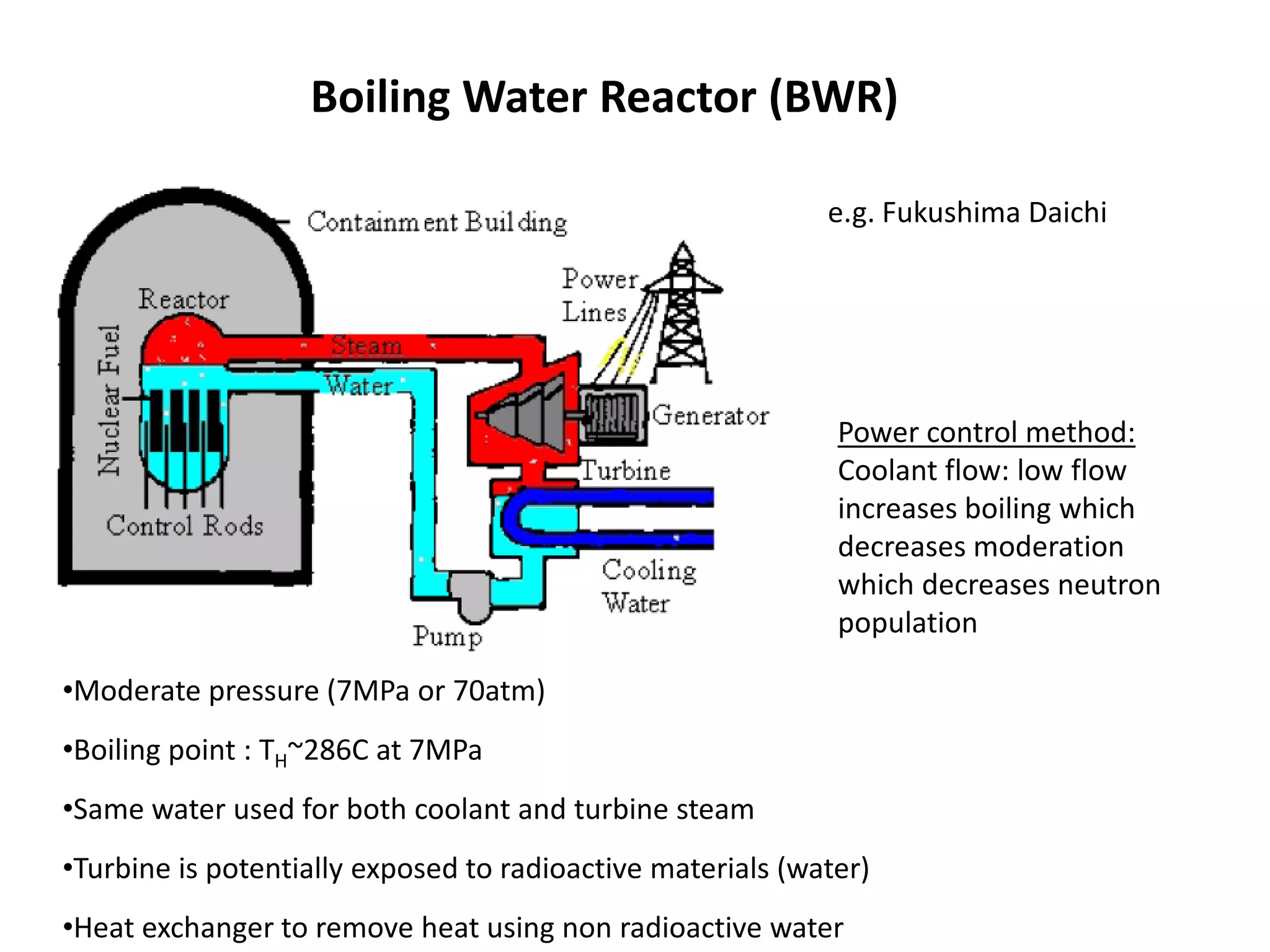
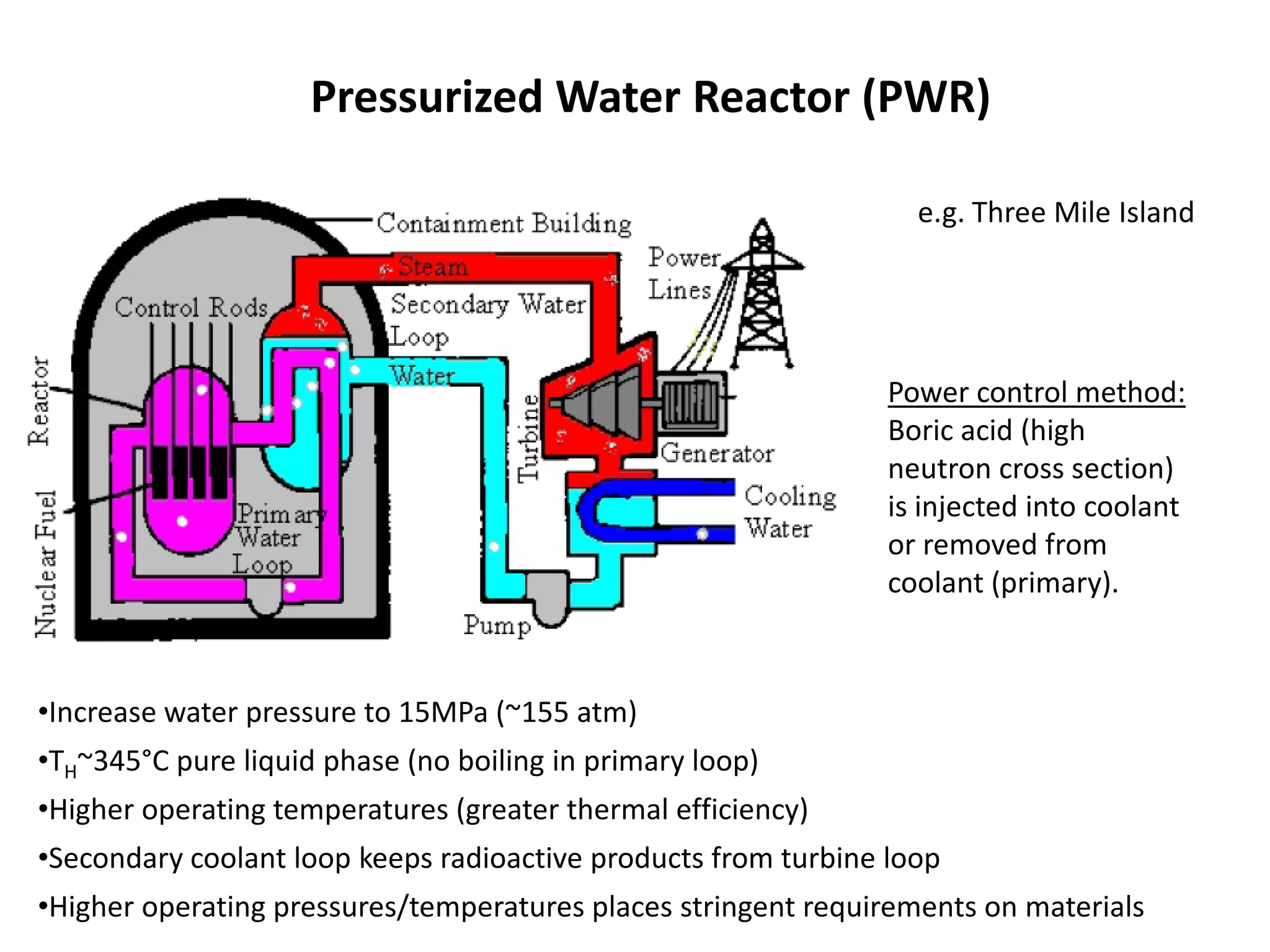
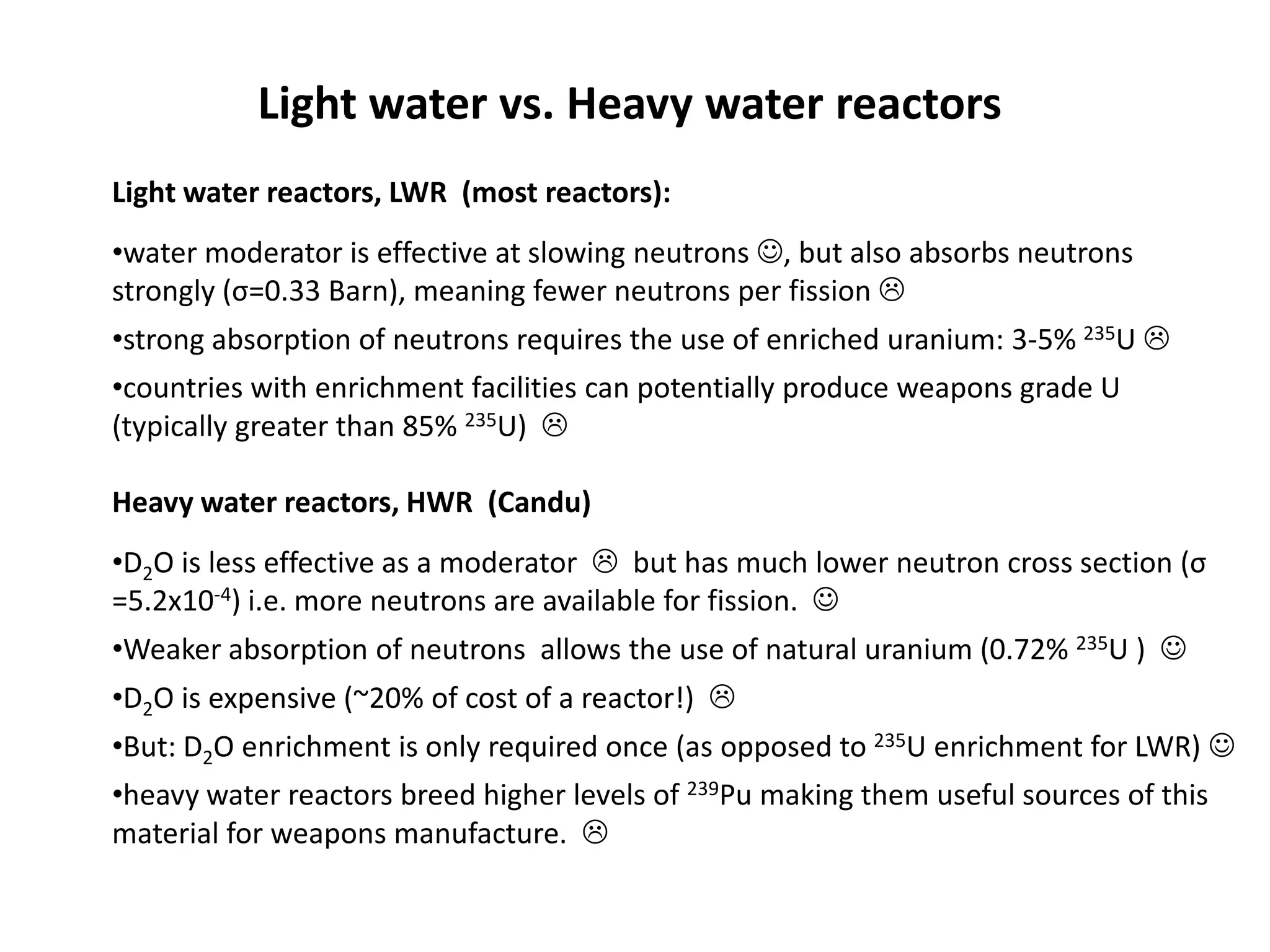
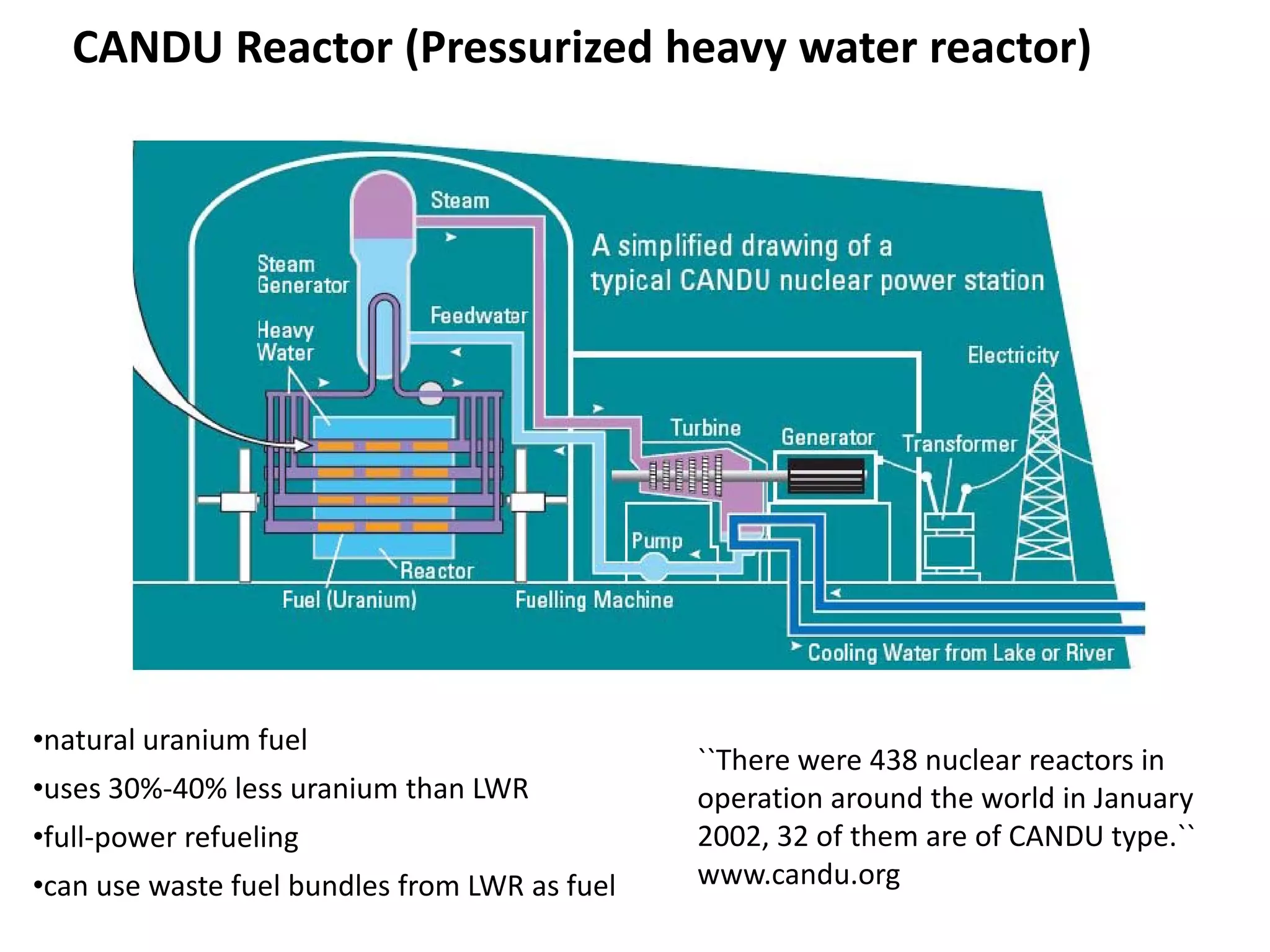
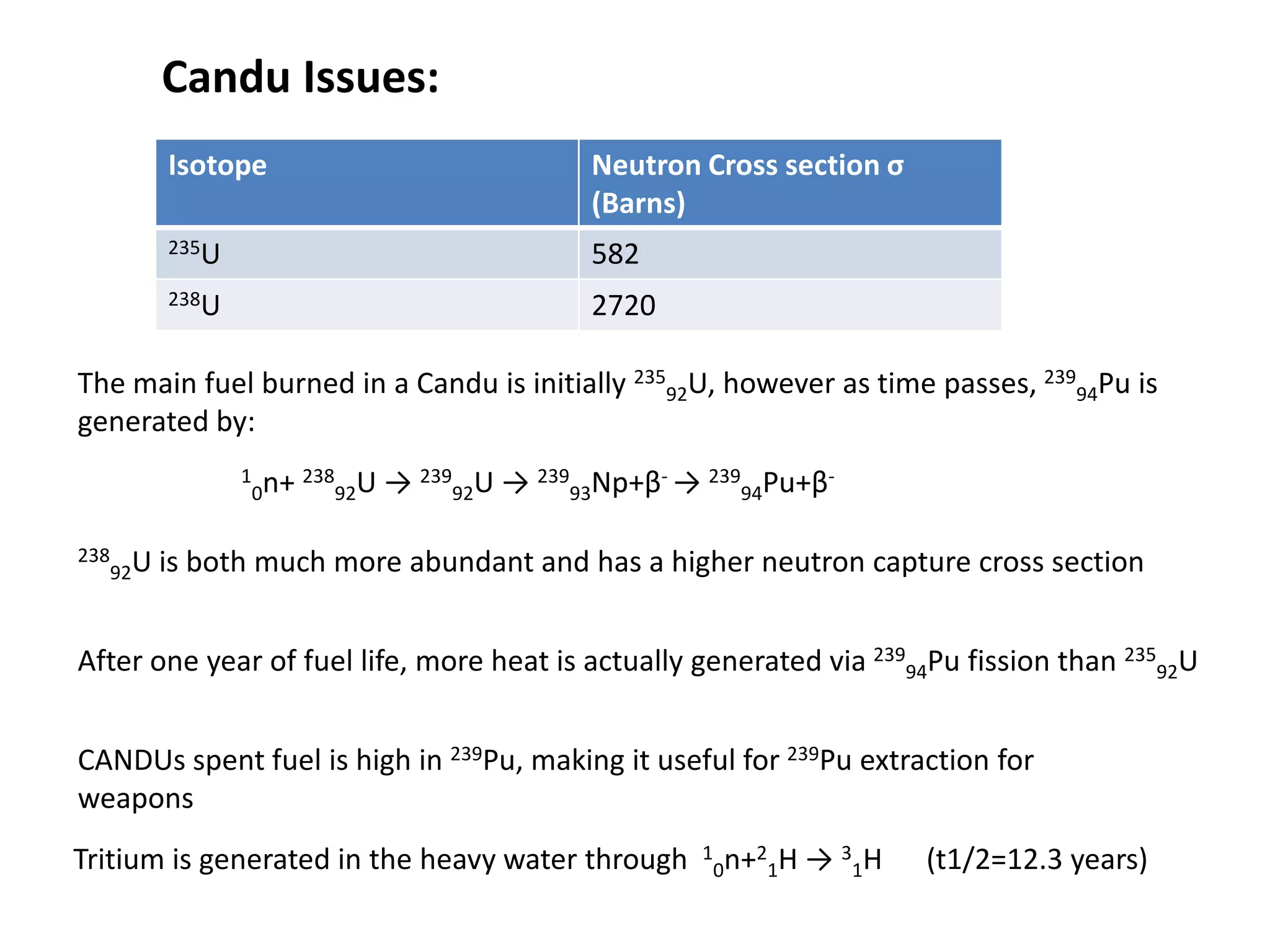
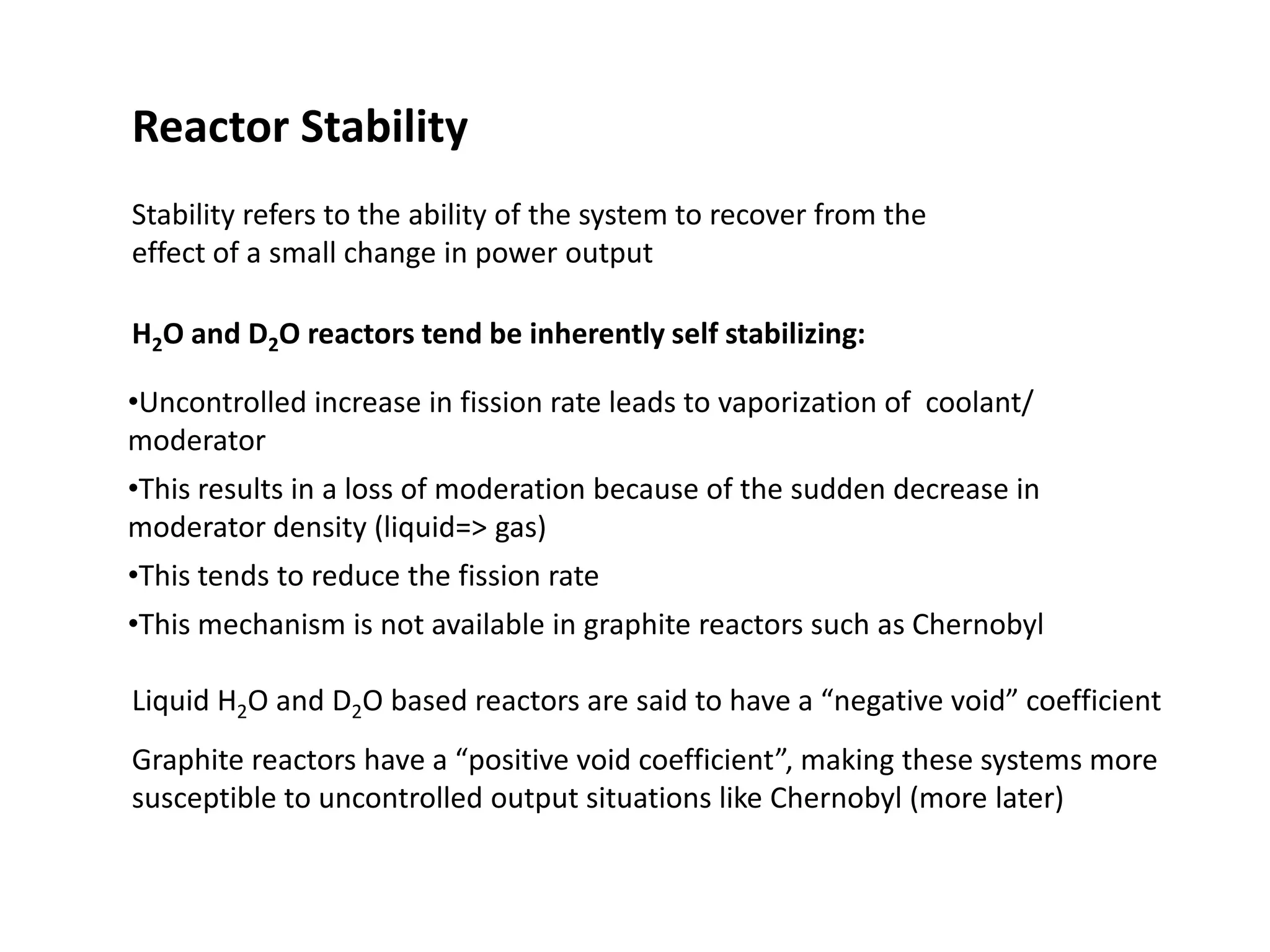
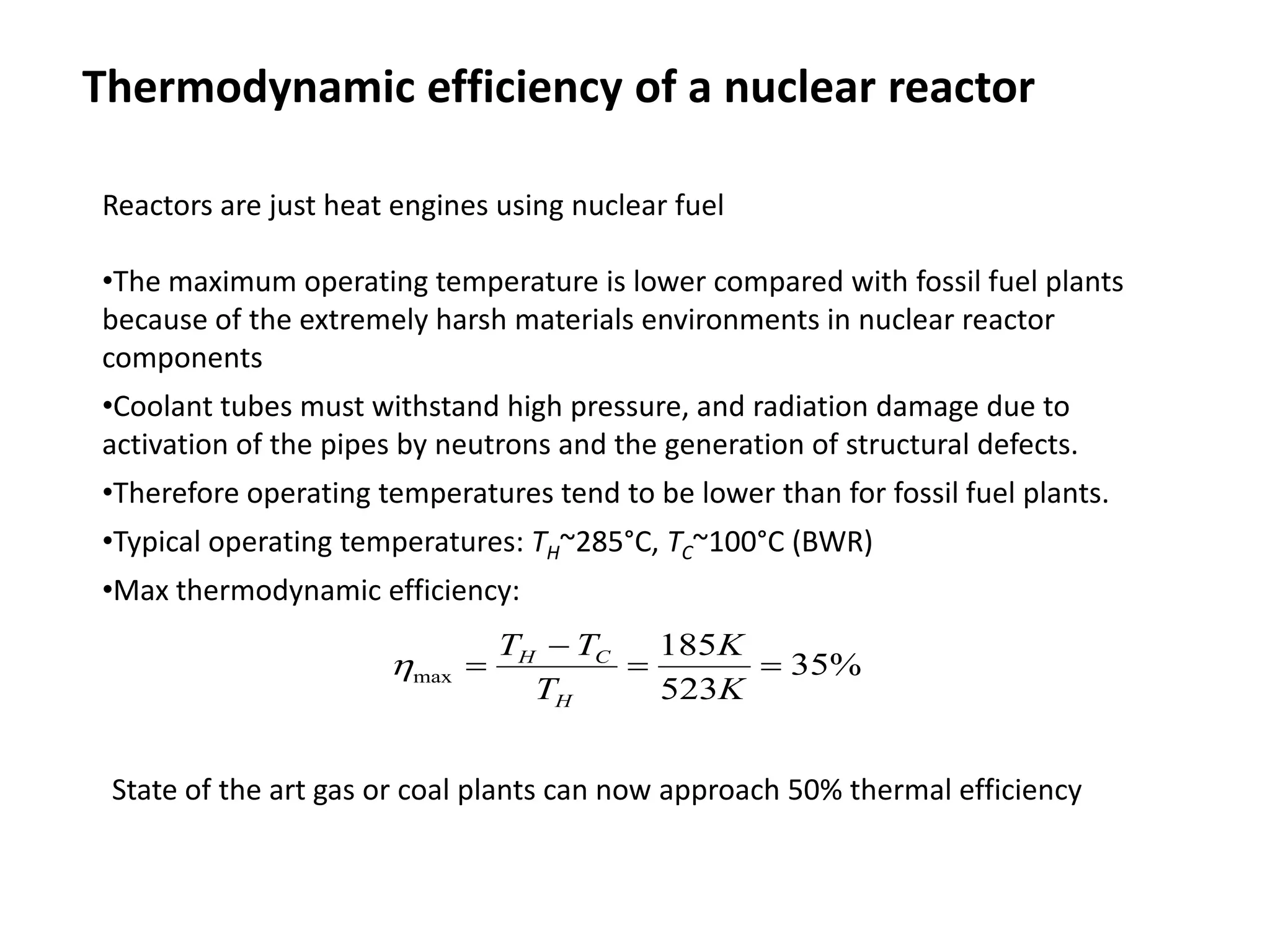
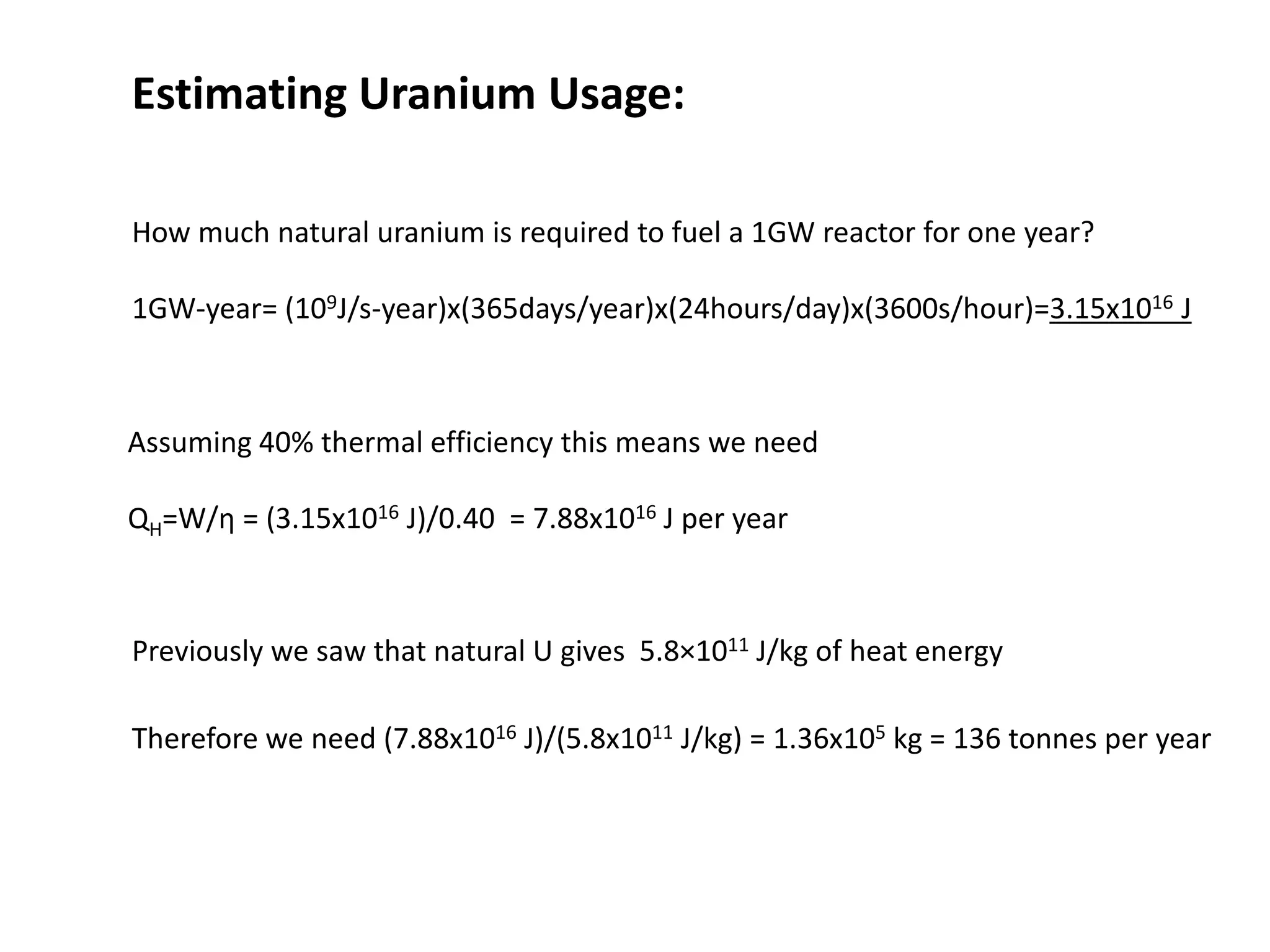
1. Nuclear reactors work by initiating a chain reaction through neutron-induced fission of uranium-235 atoms, releasing energy and more neutrons.
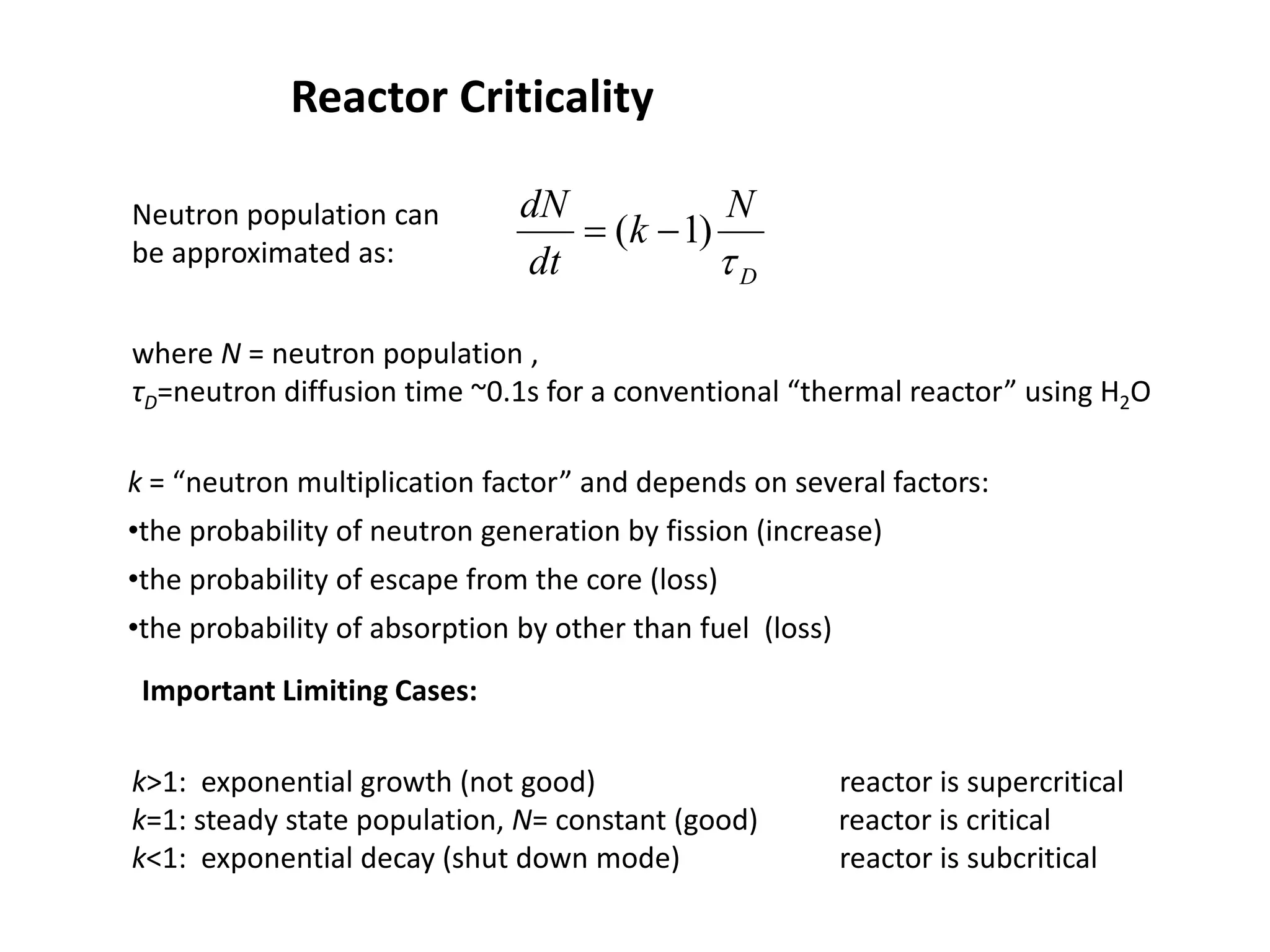
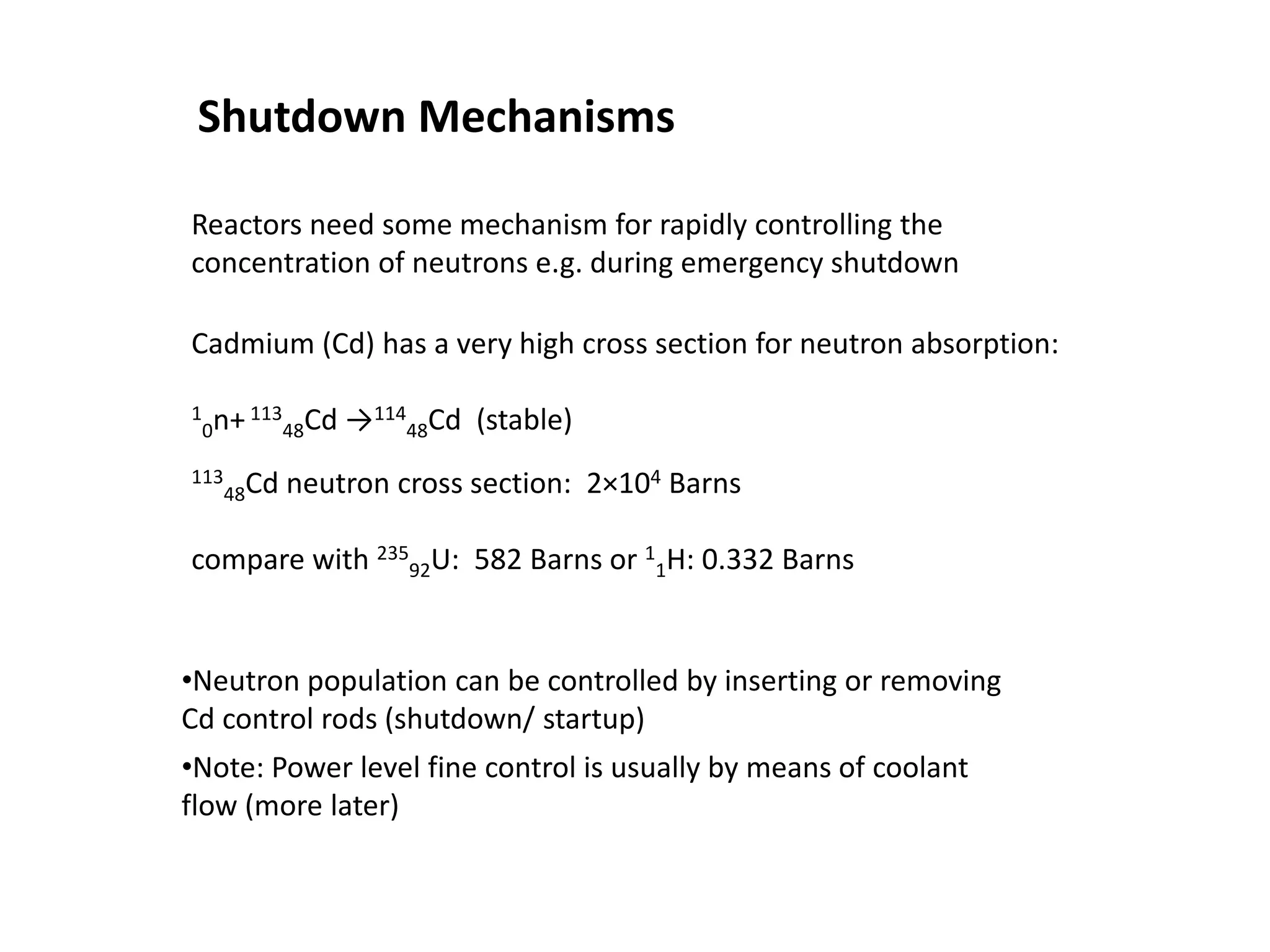
2. Neutrons are slowed by a moderator like water or graphite to sustain the chain reaction. The reaction is controlled by absorbing excess neutrons or changing moderator conditions.
3. Heat from fission is used in a heat exchanger to power turbines for electricity generation.