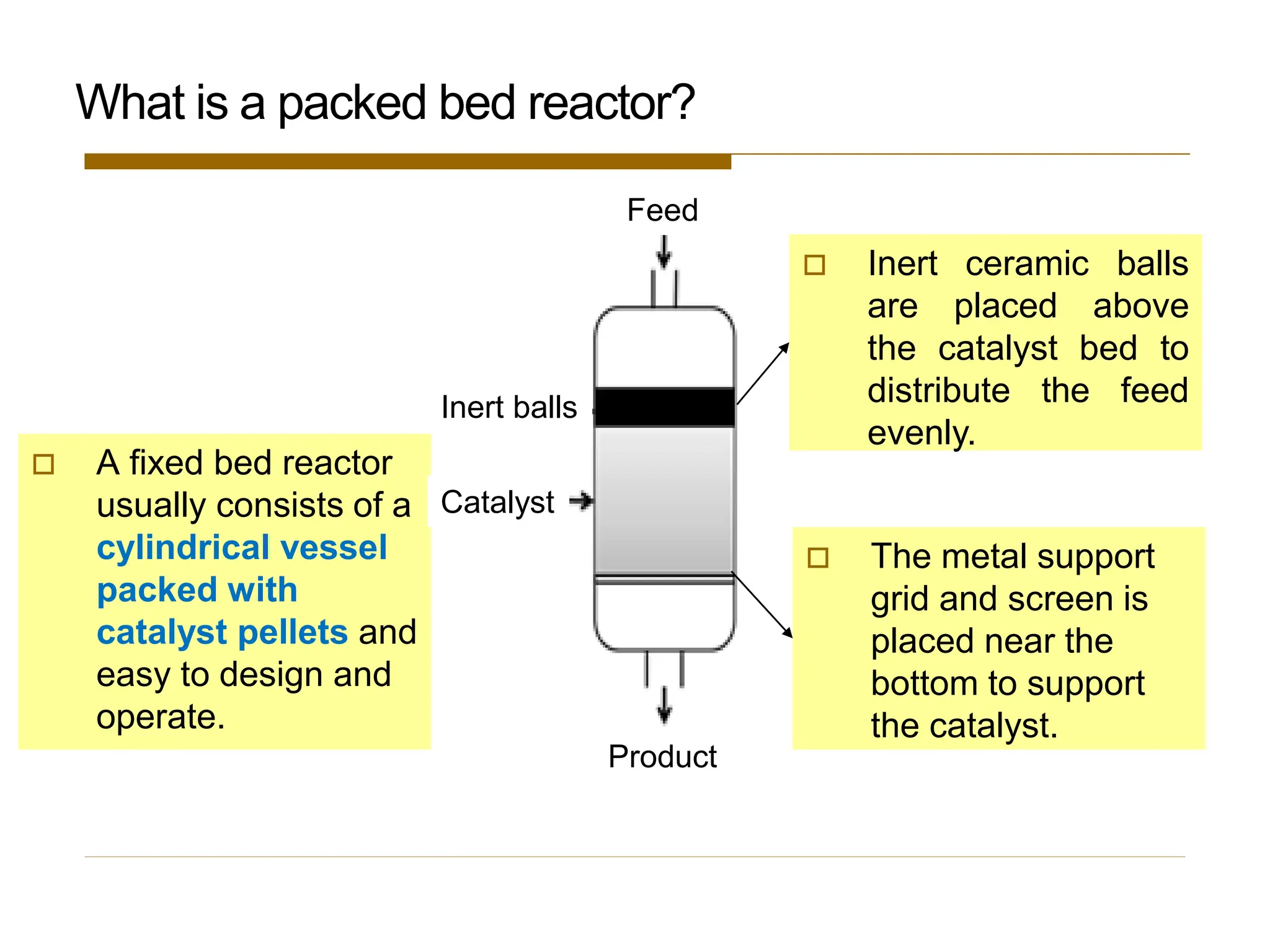
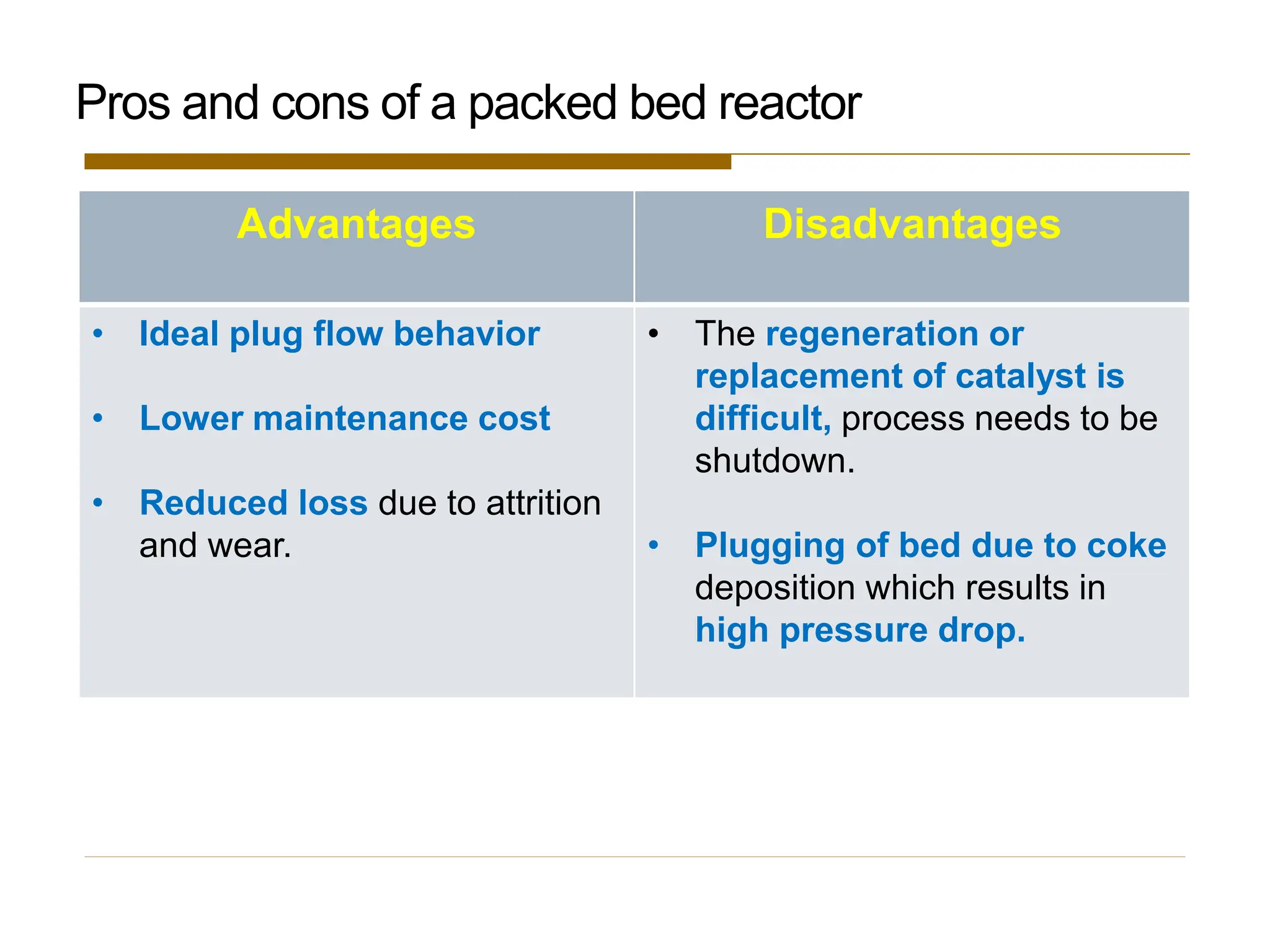
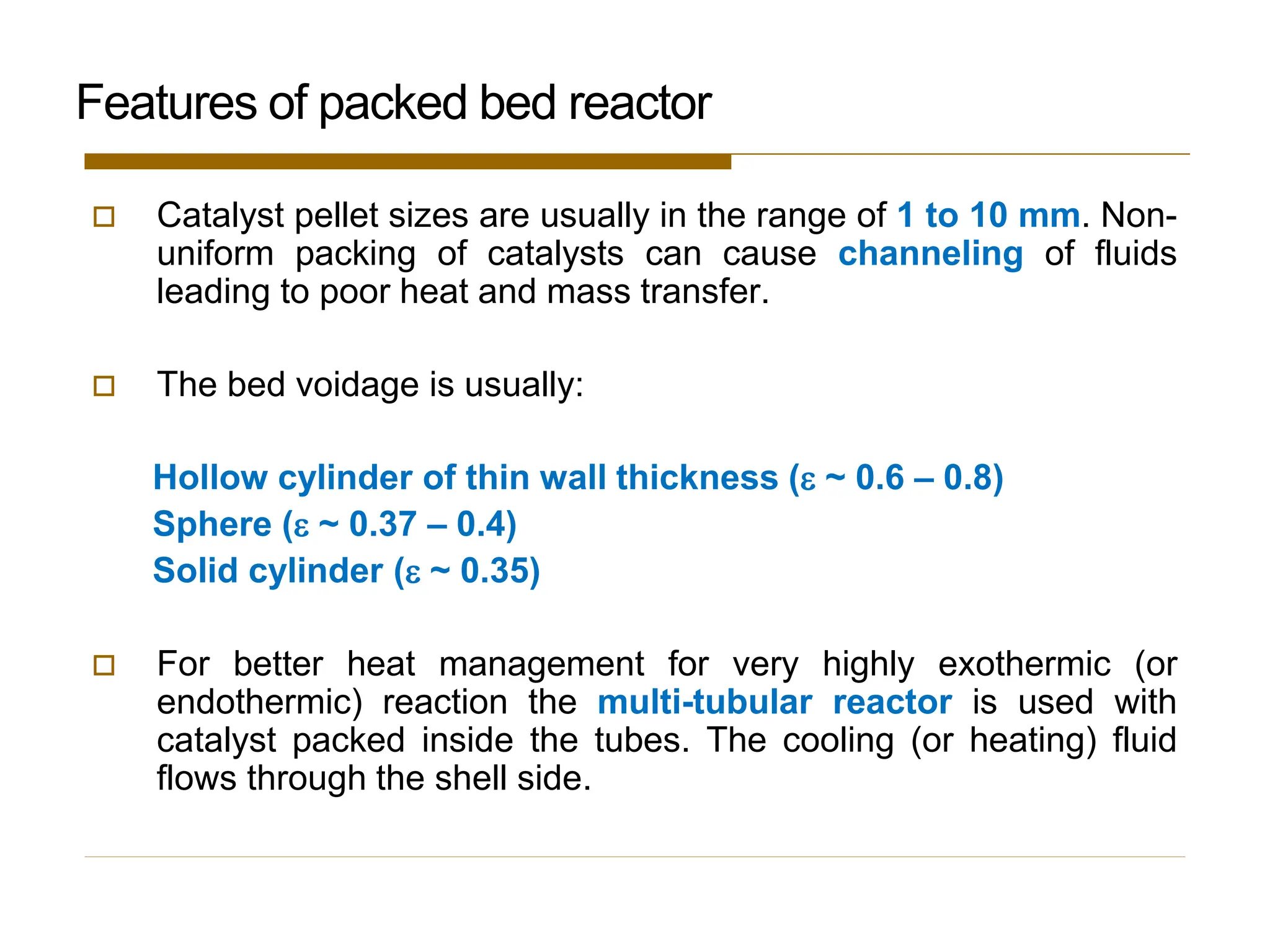
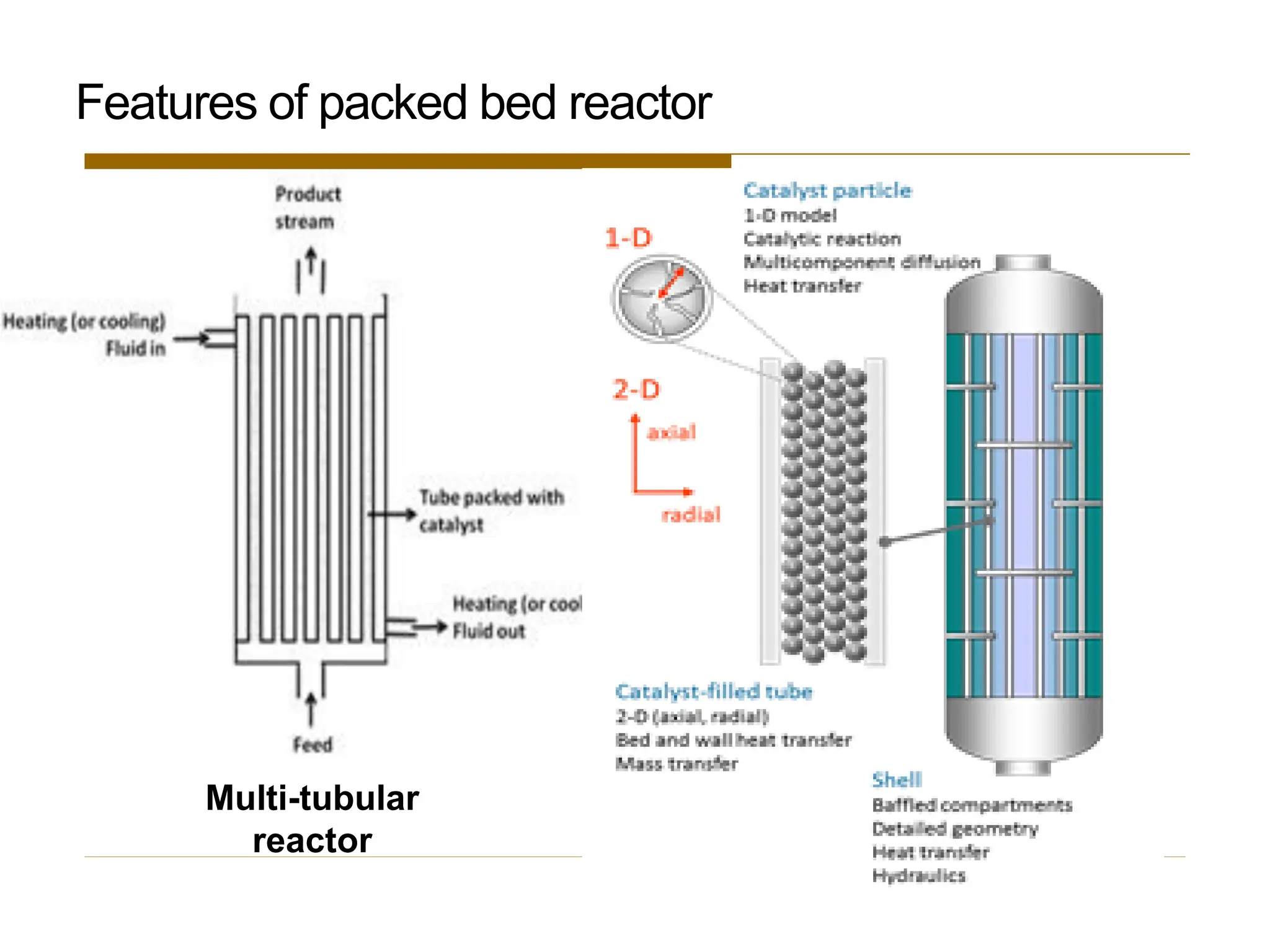
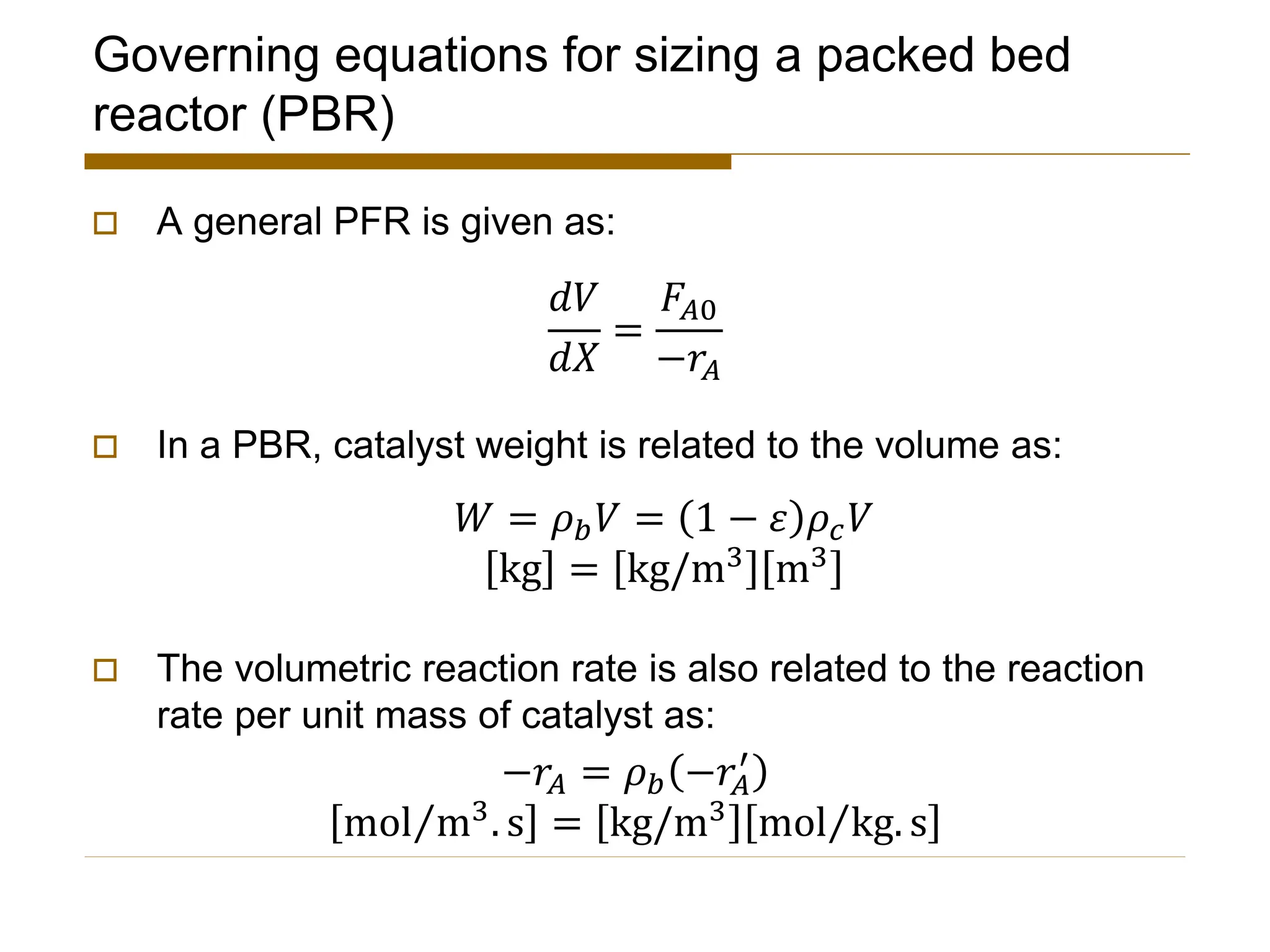
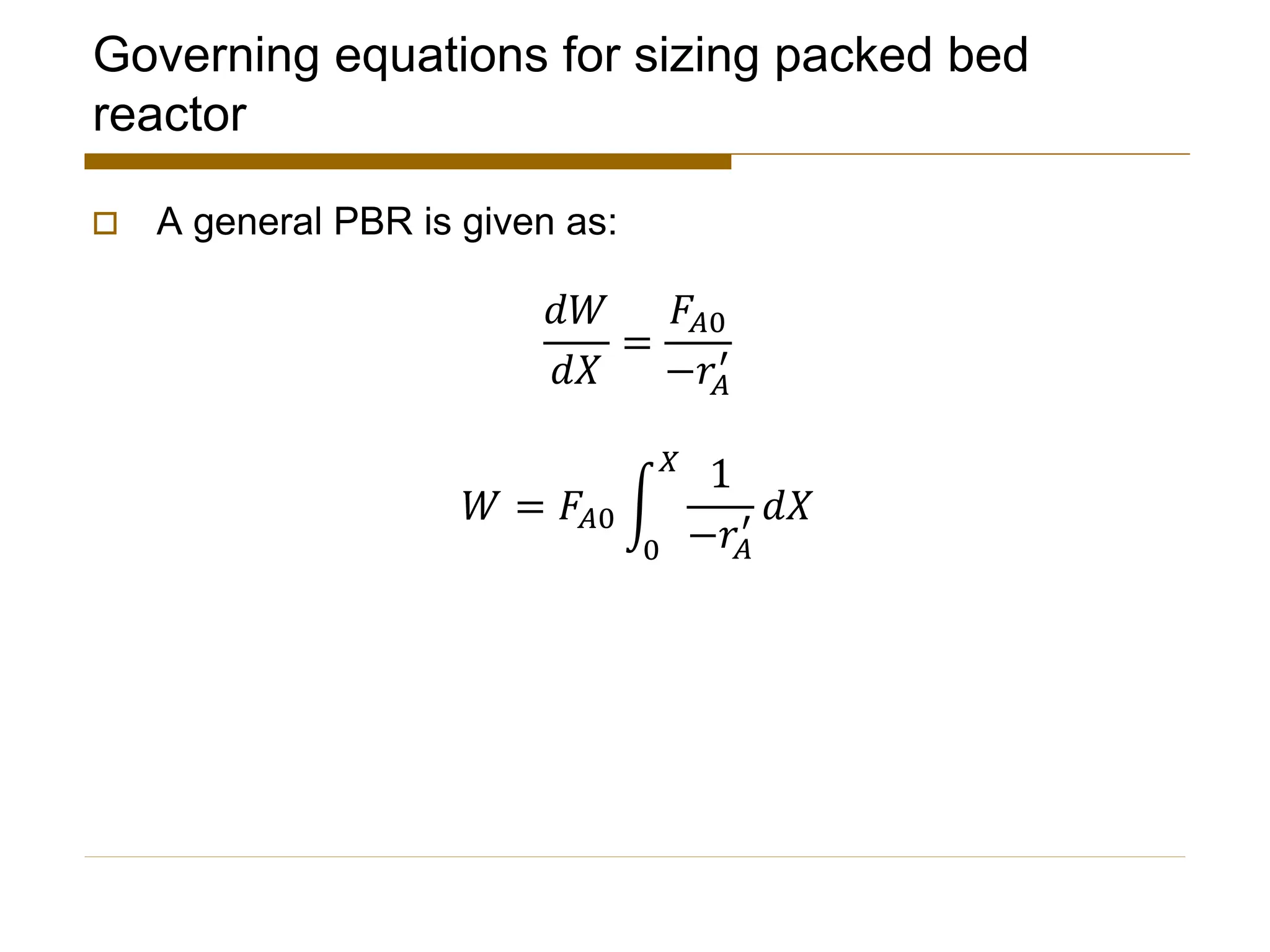
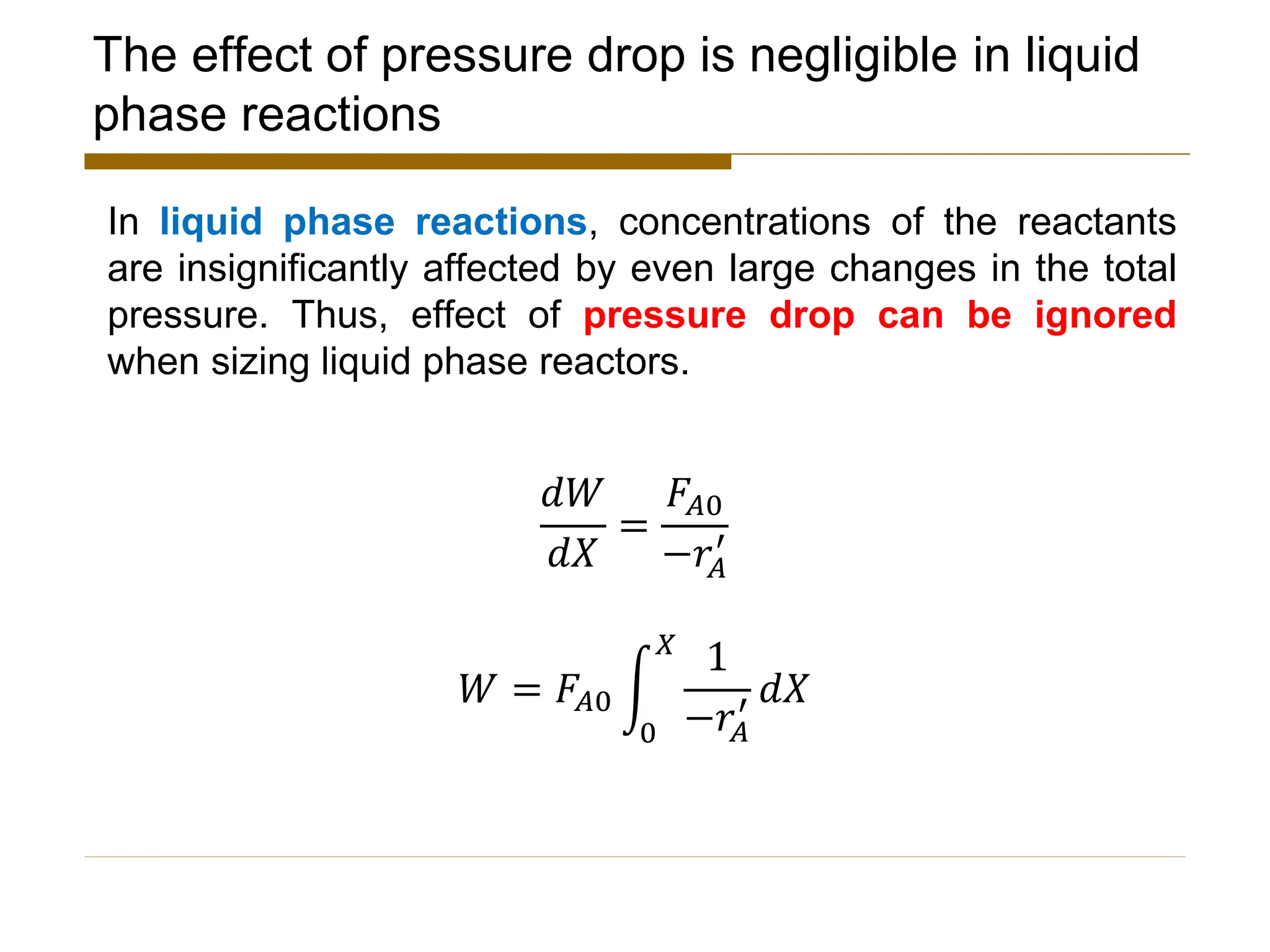
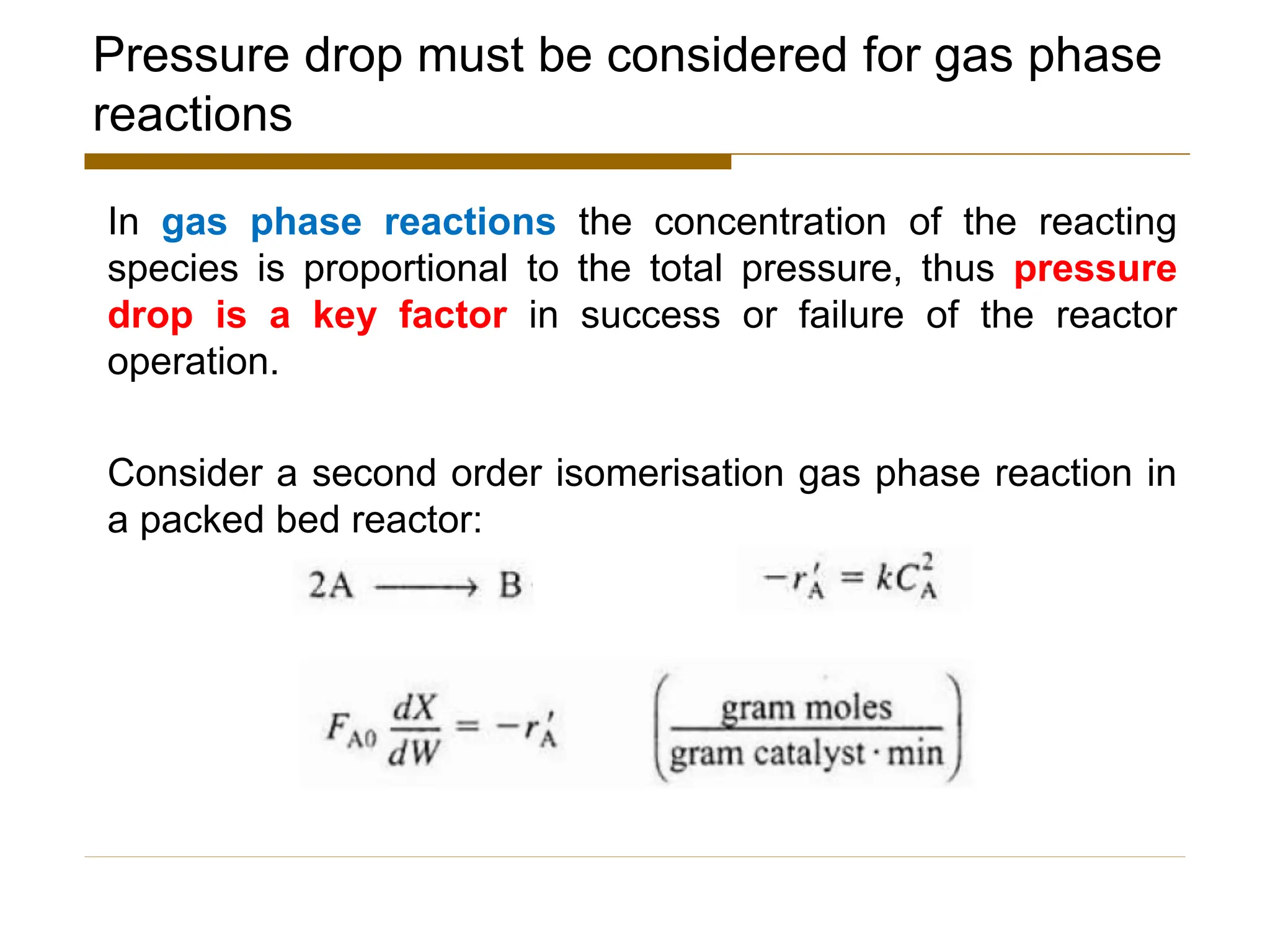
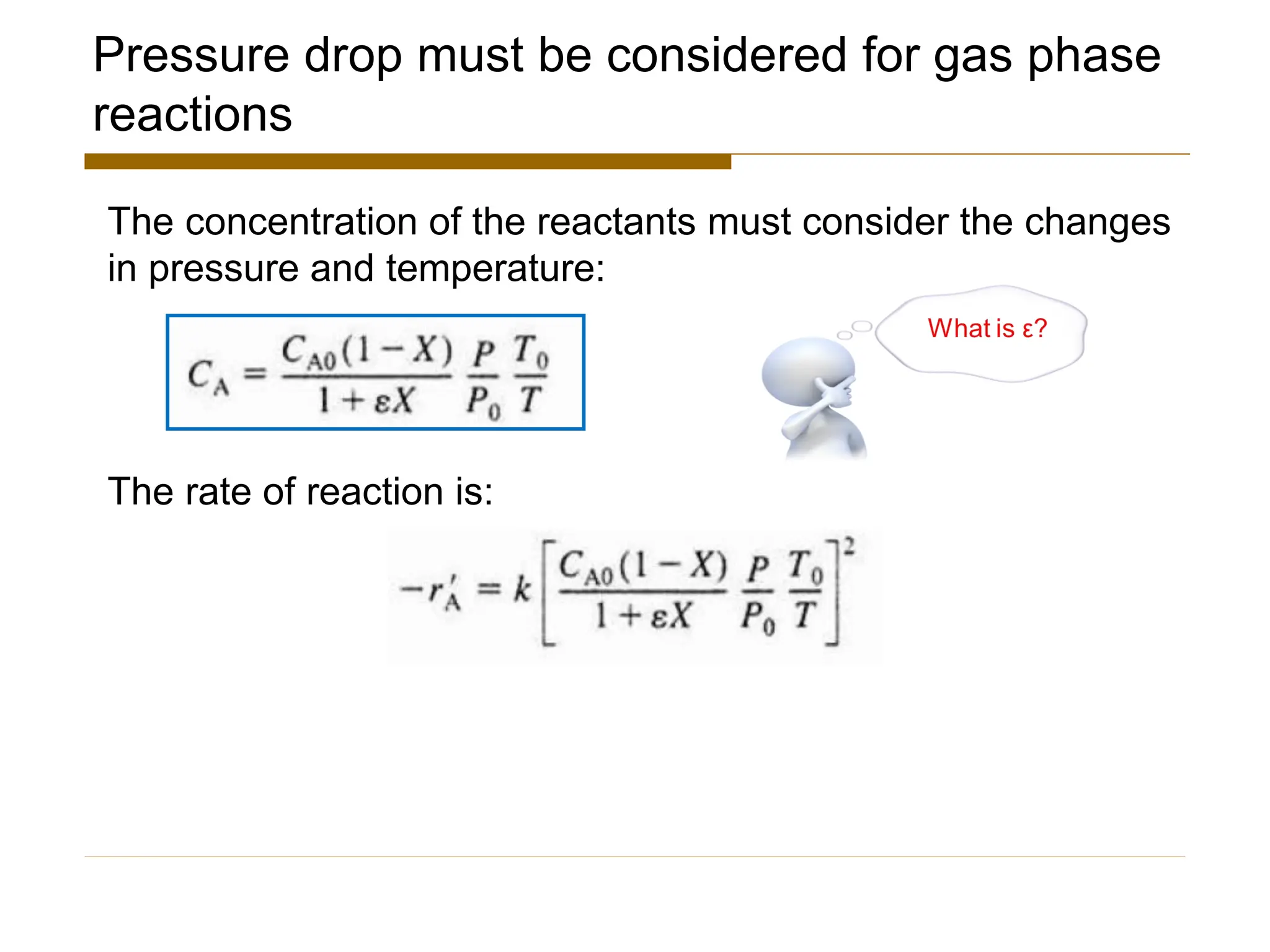
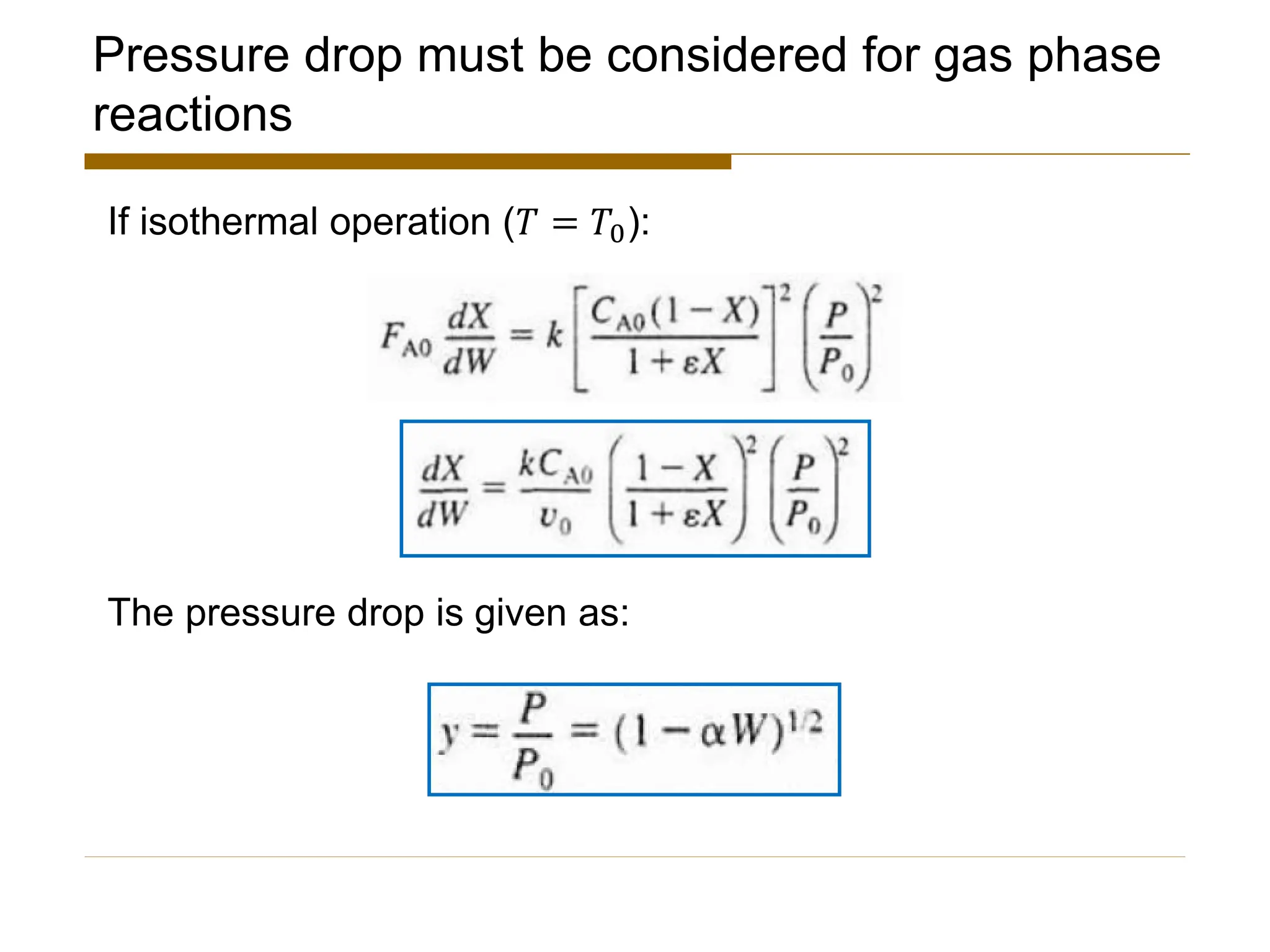
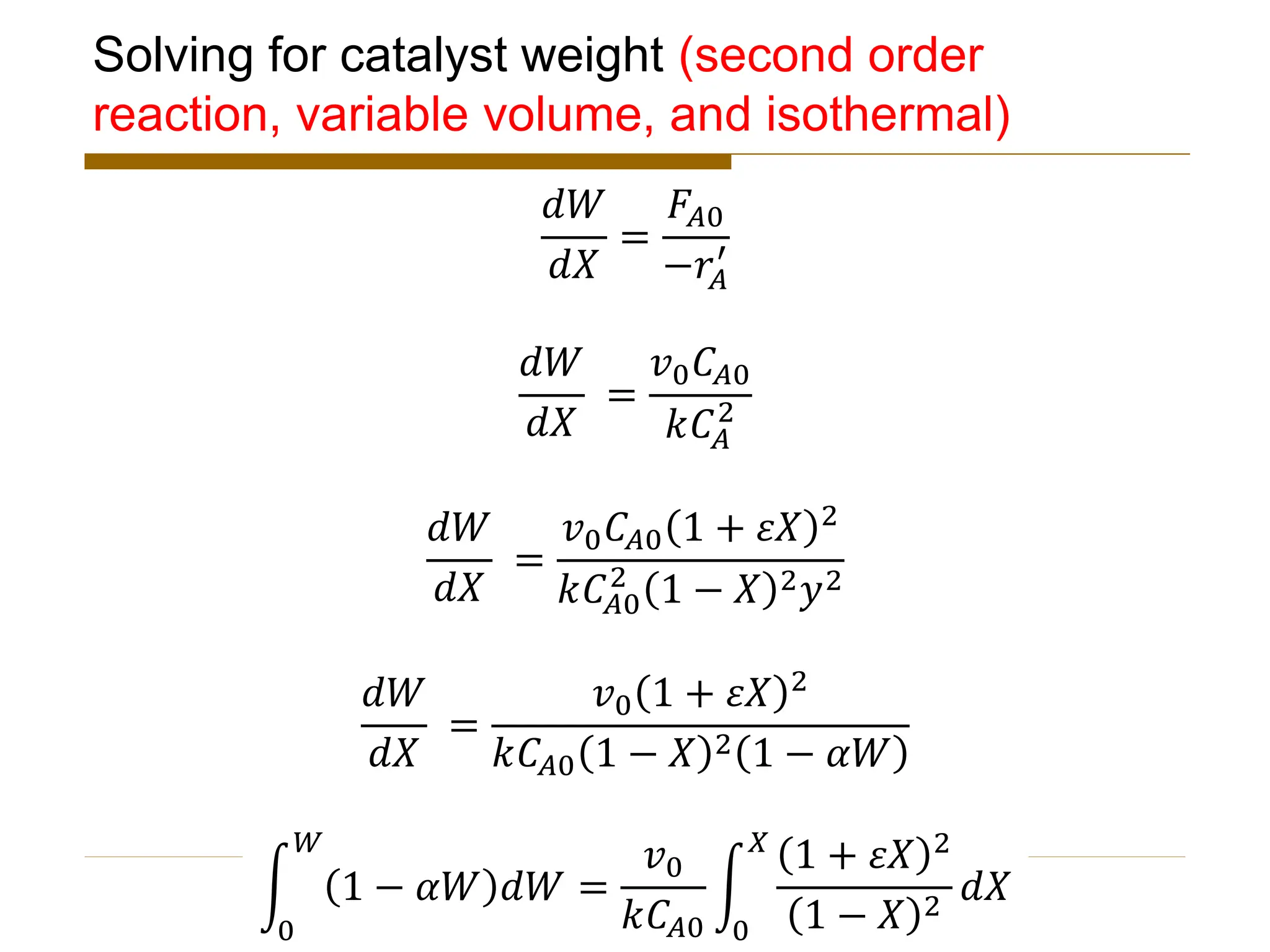
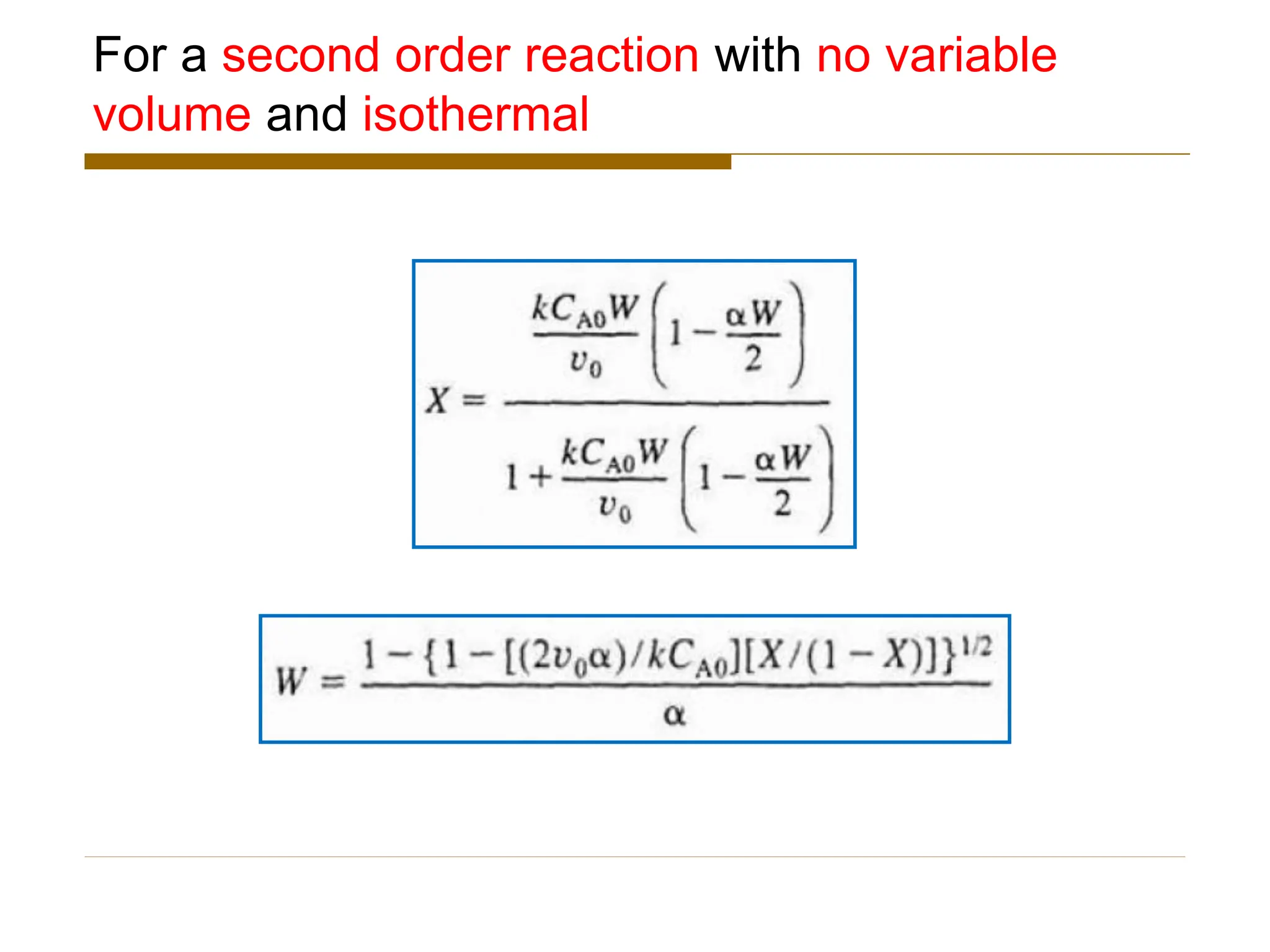
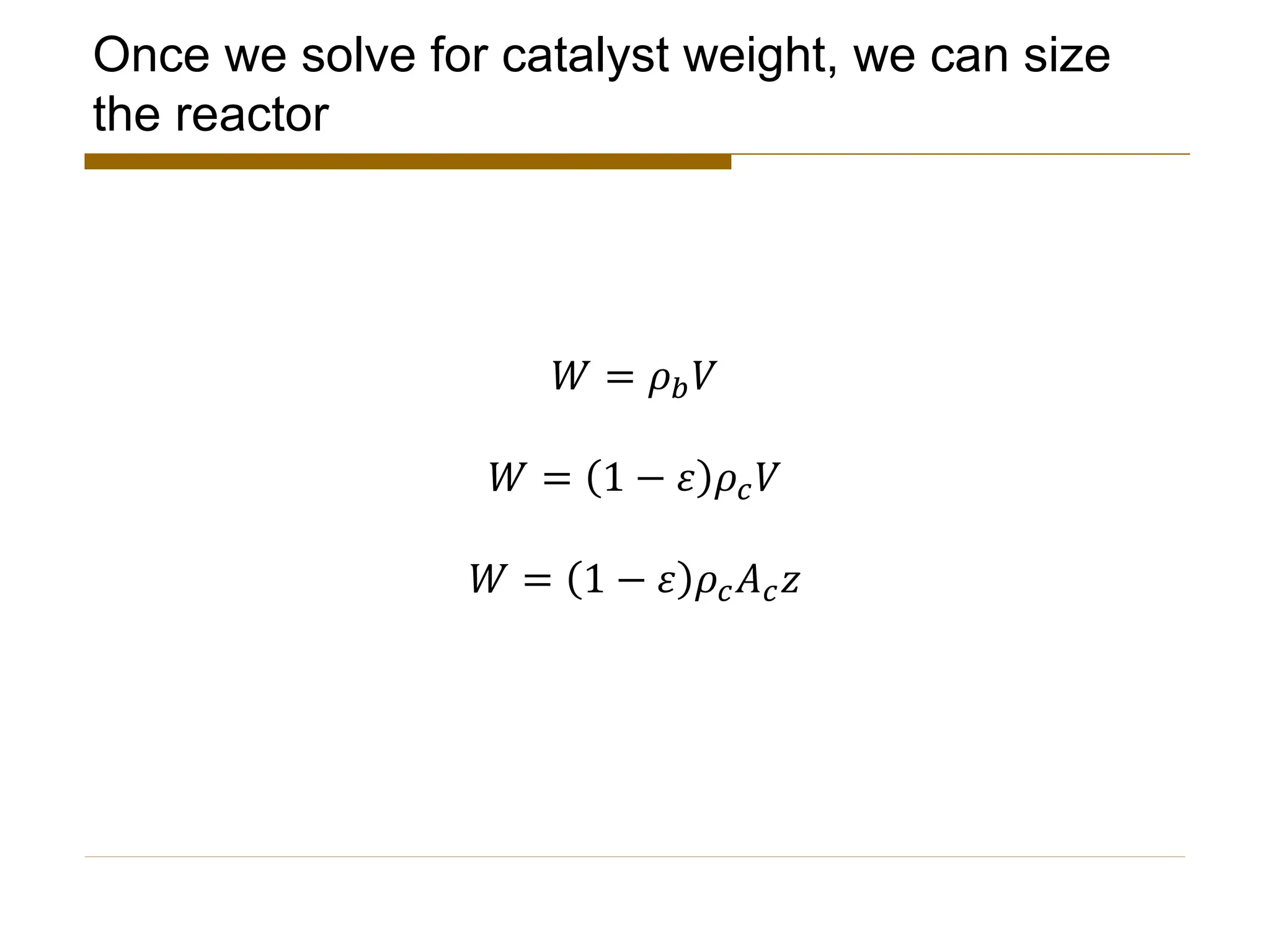
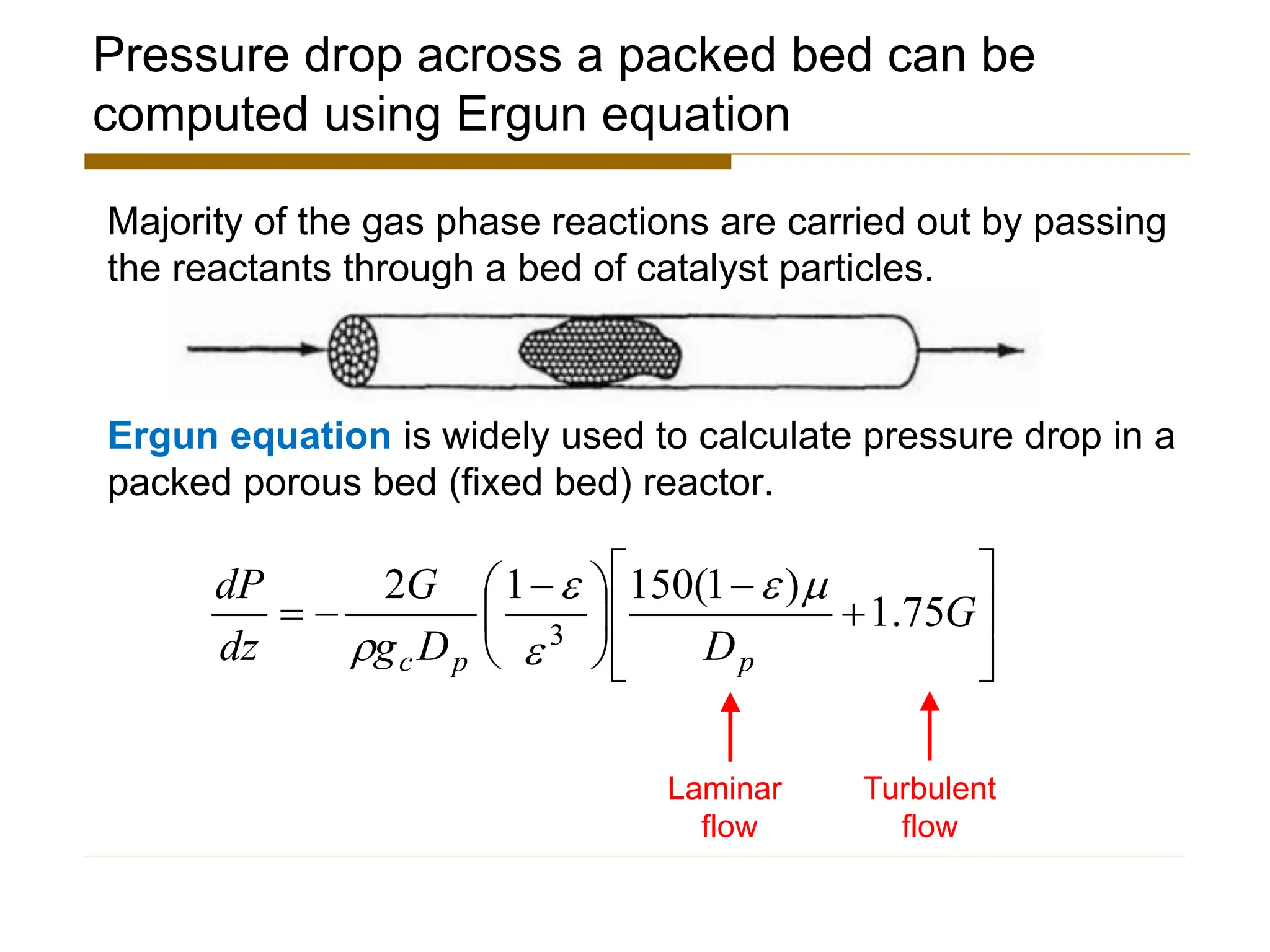
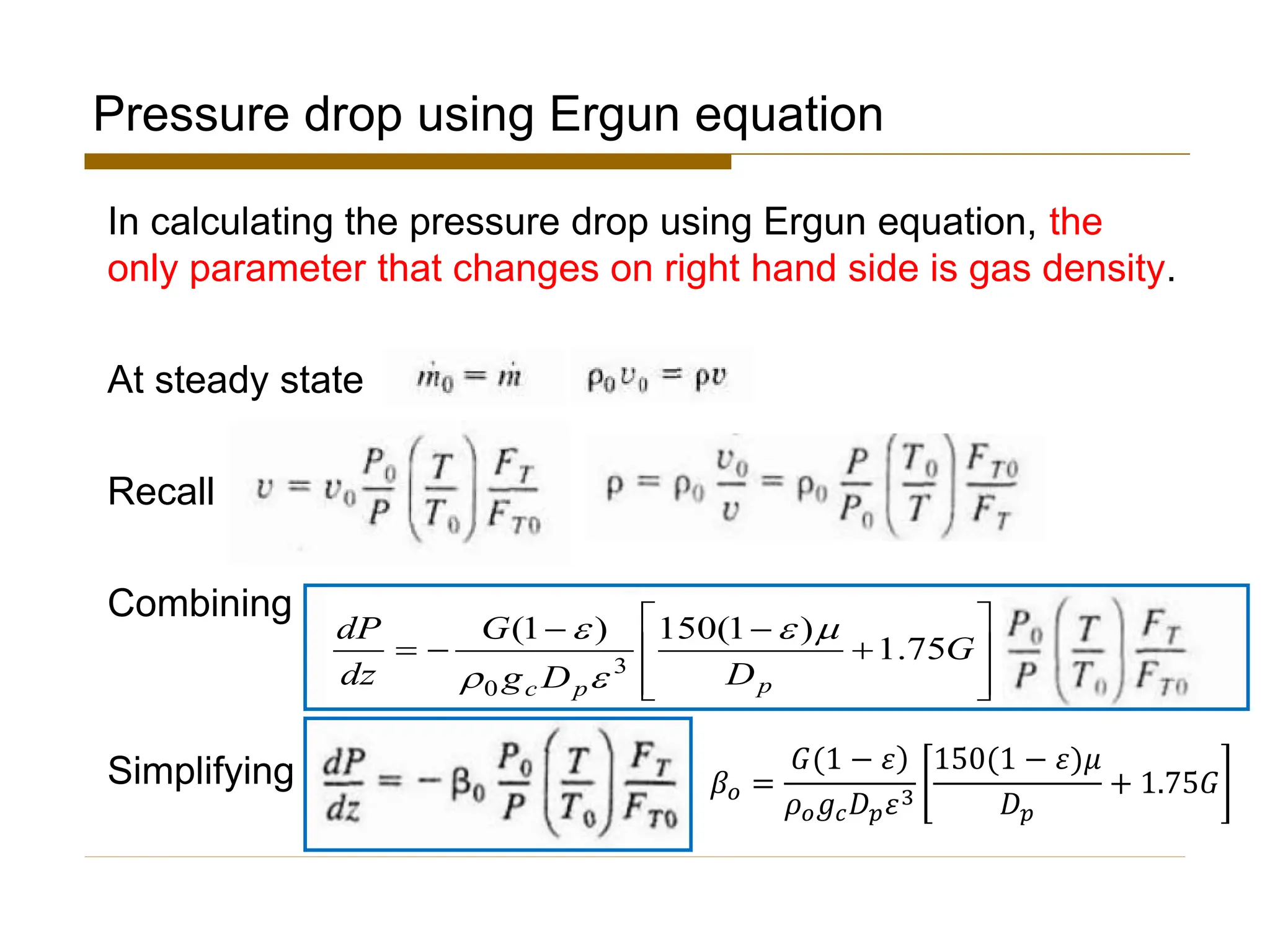
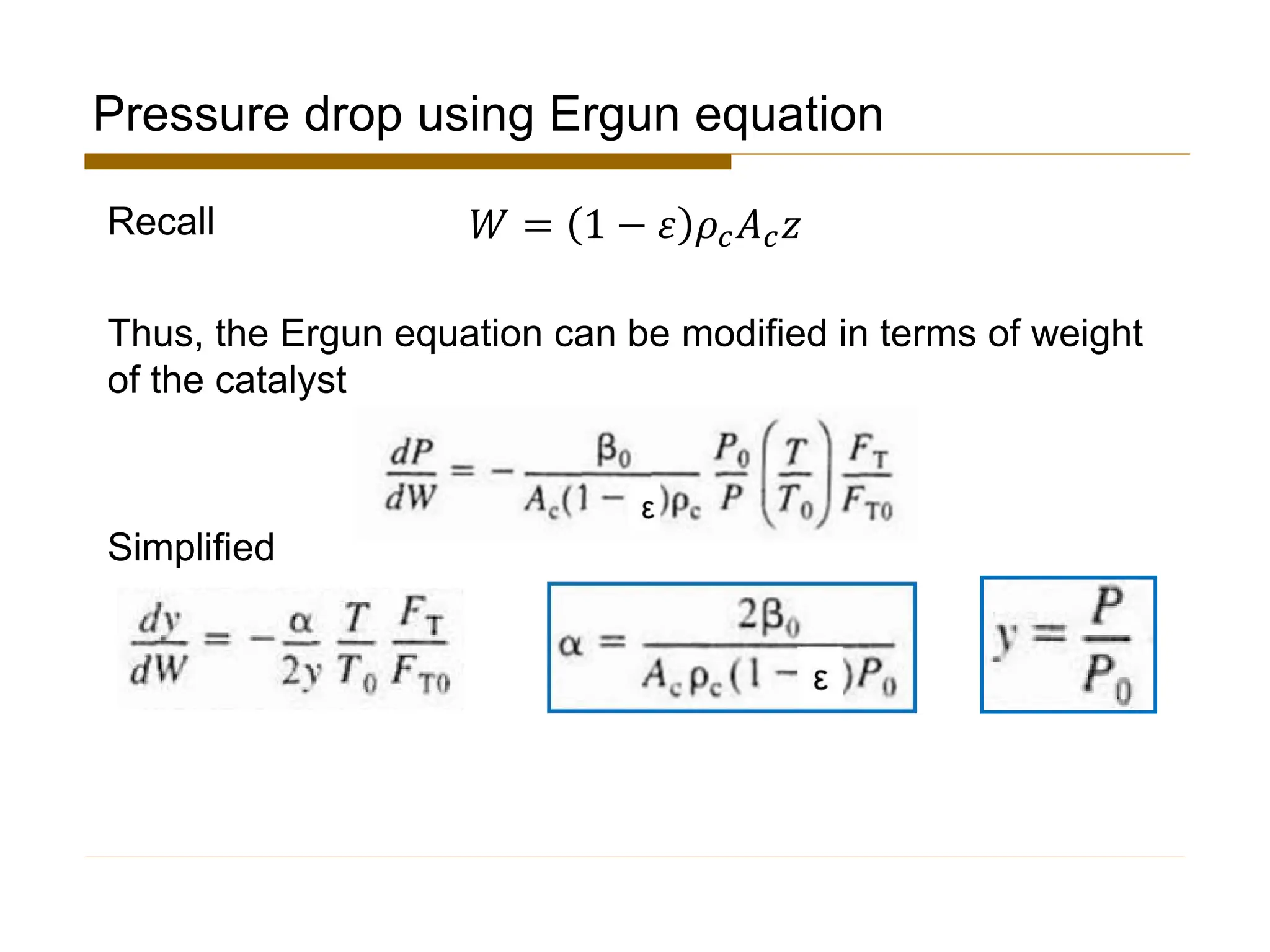
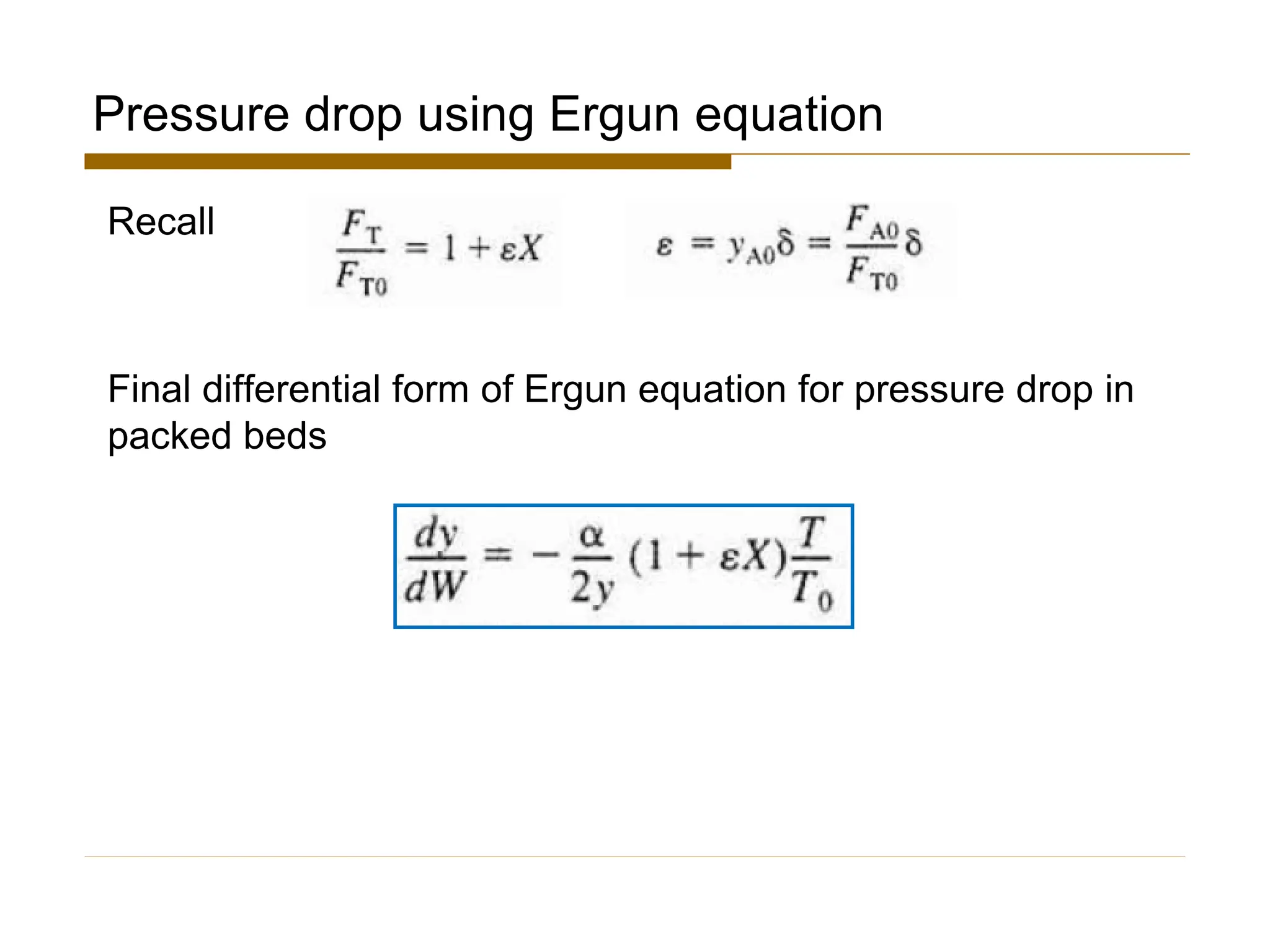
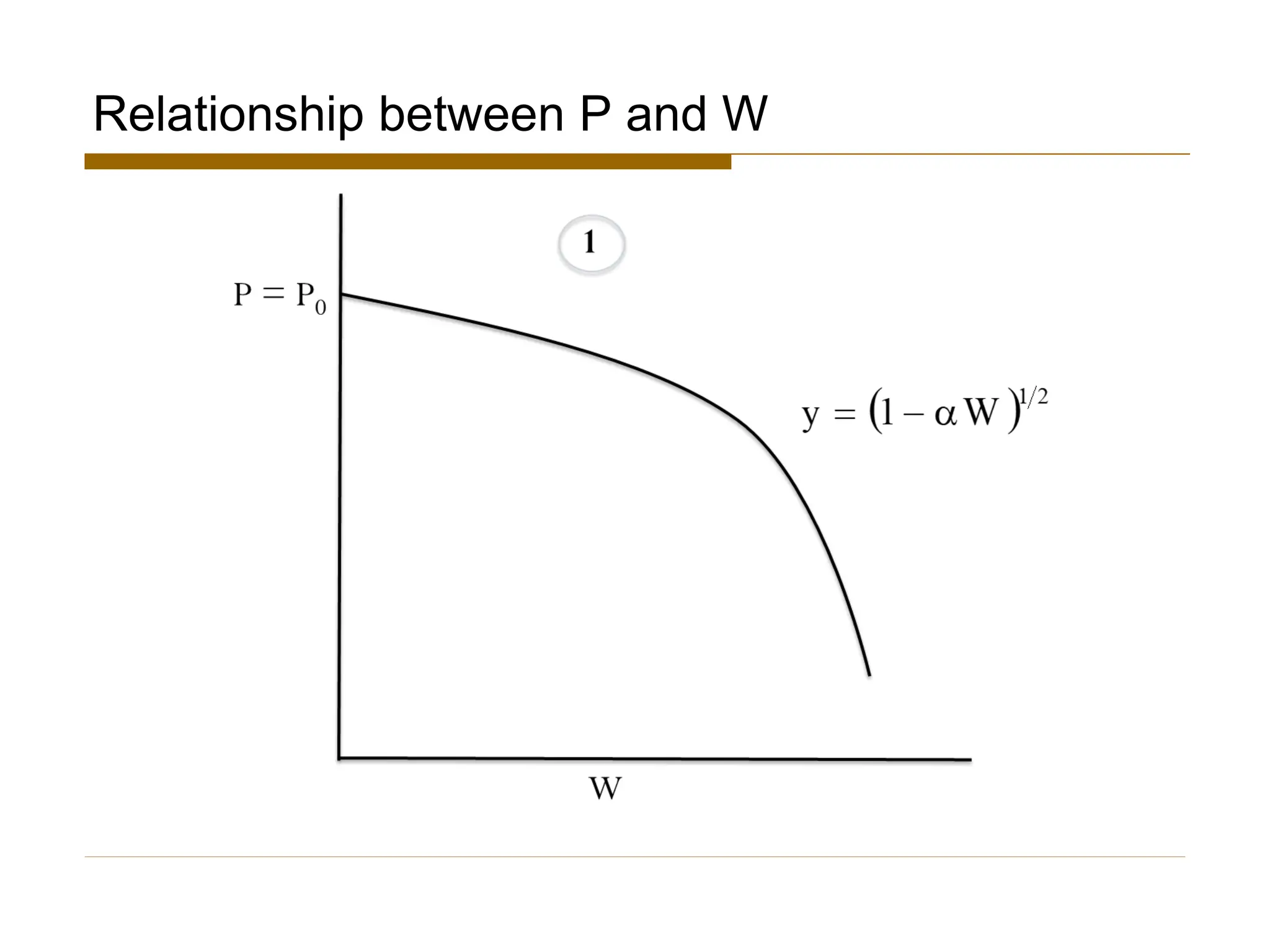
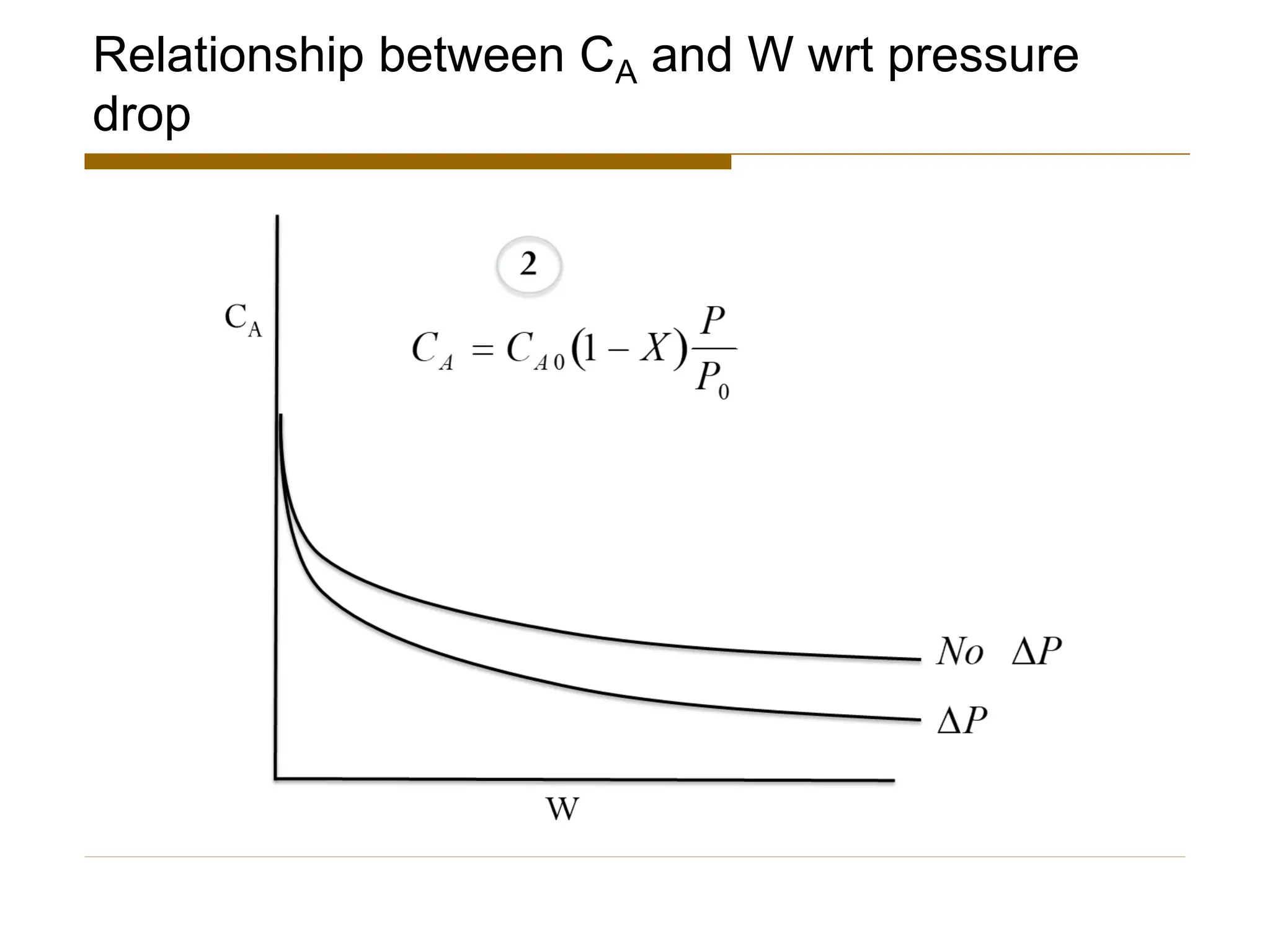
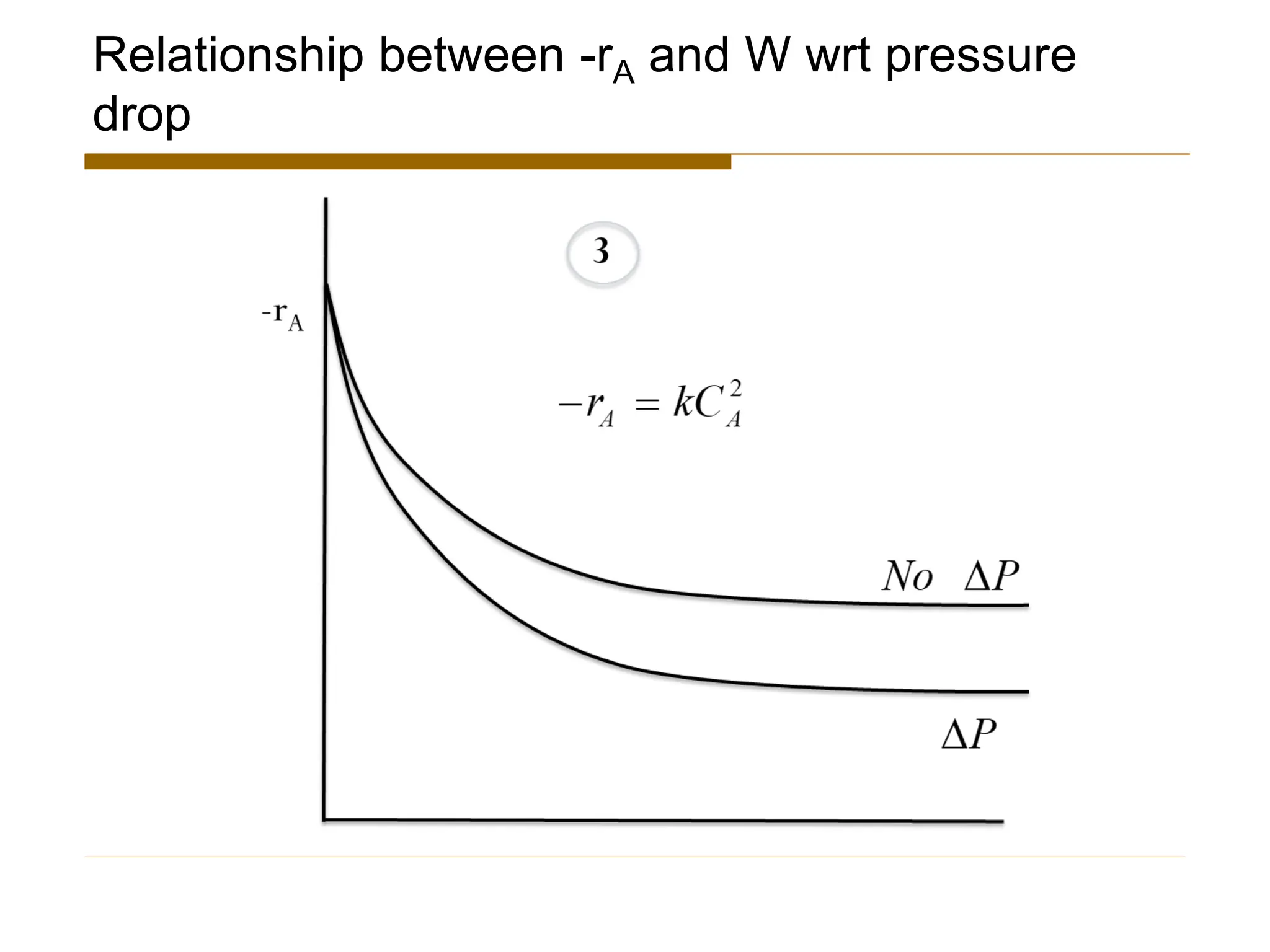
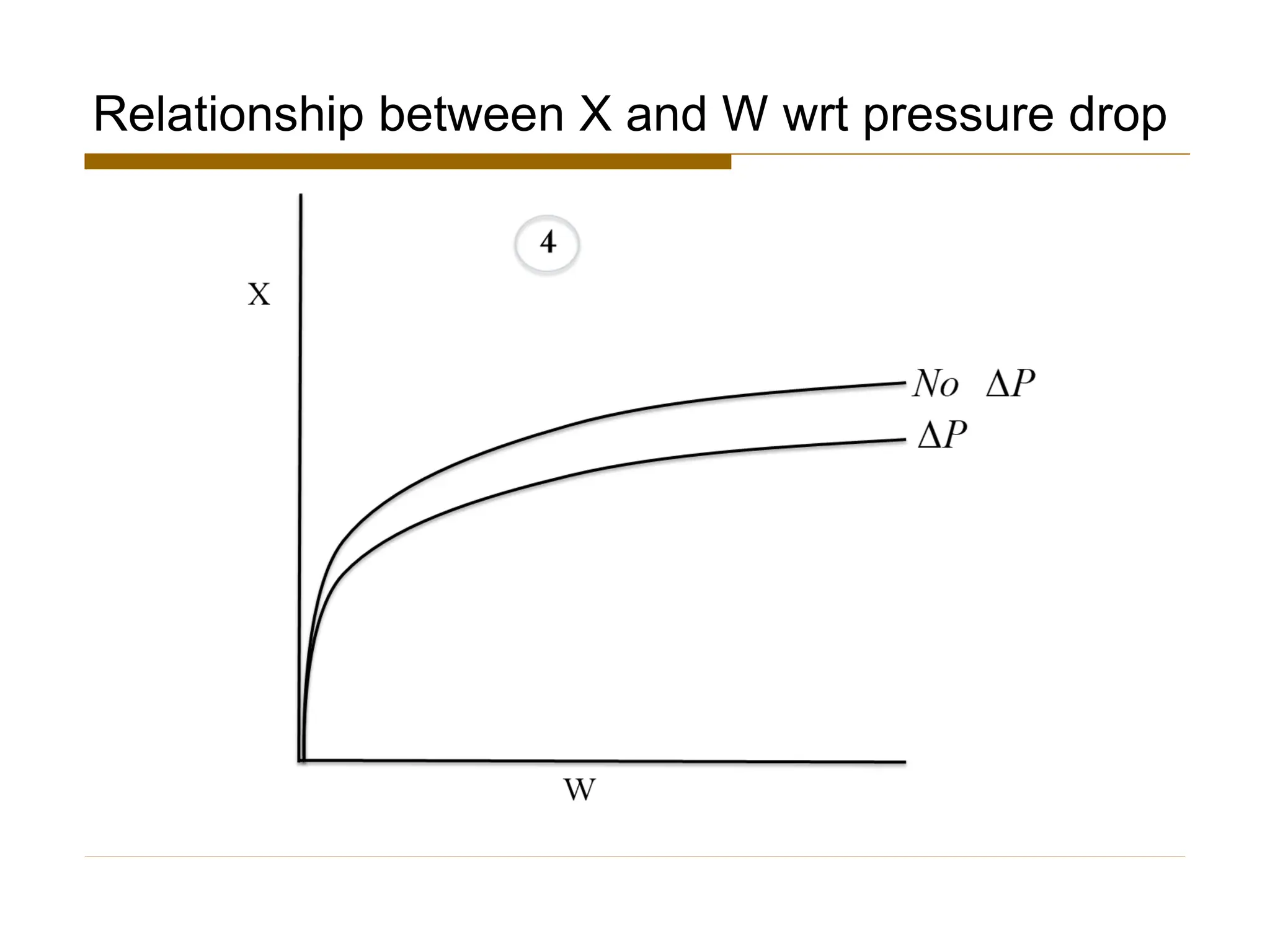
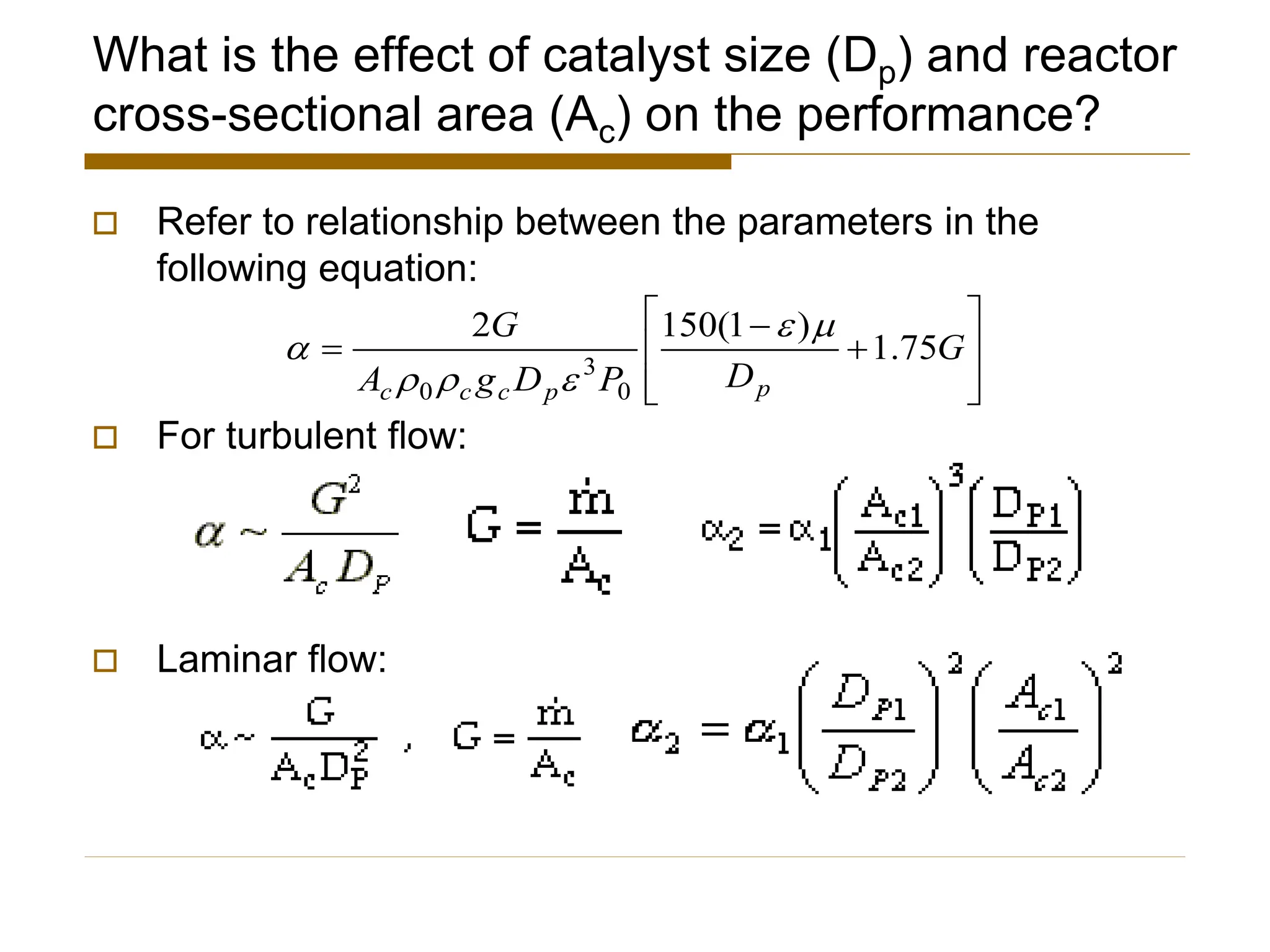
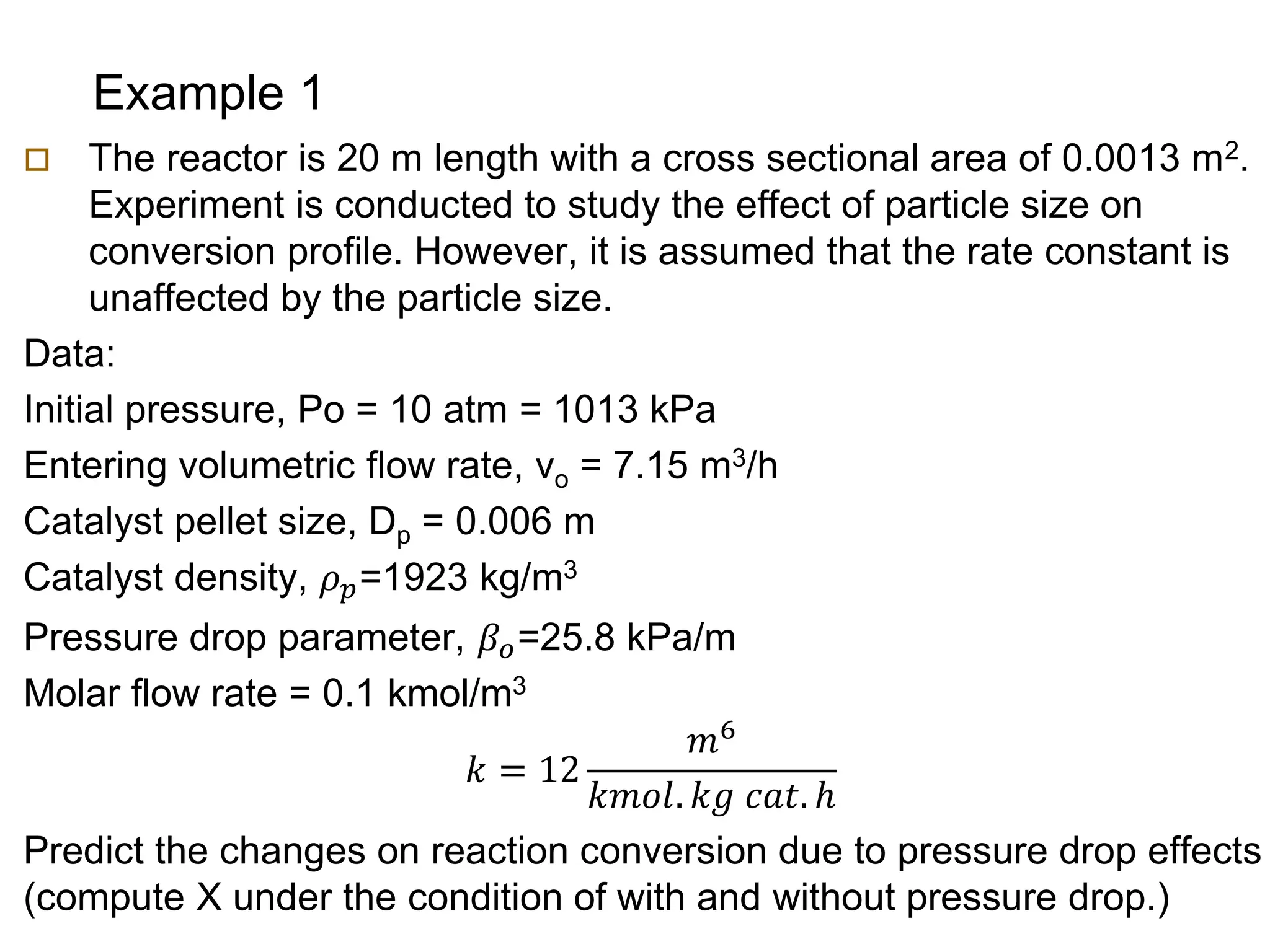
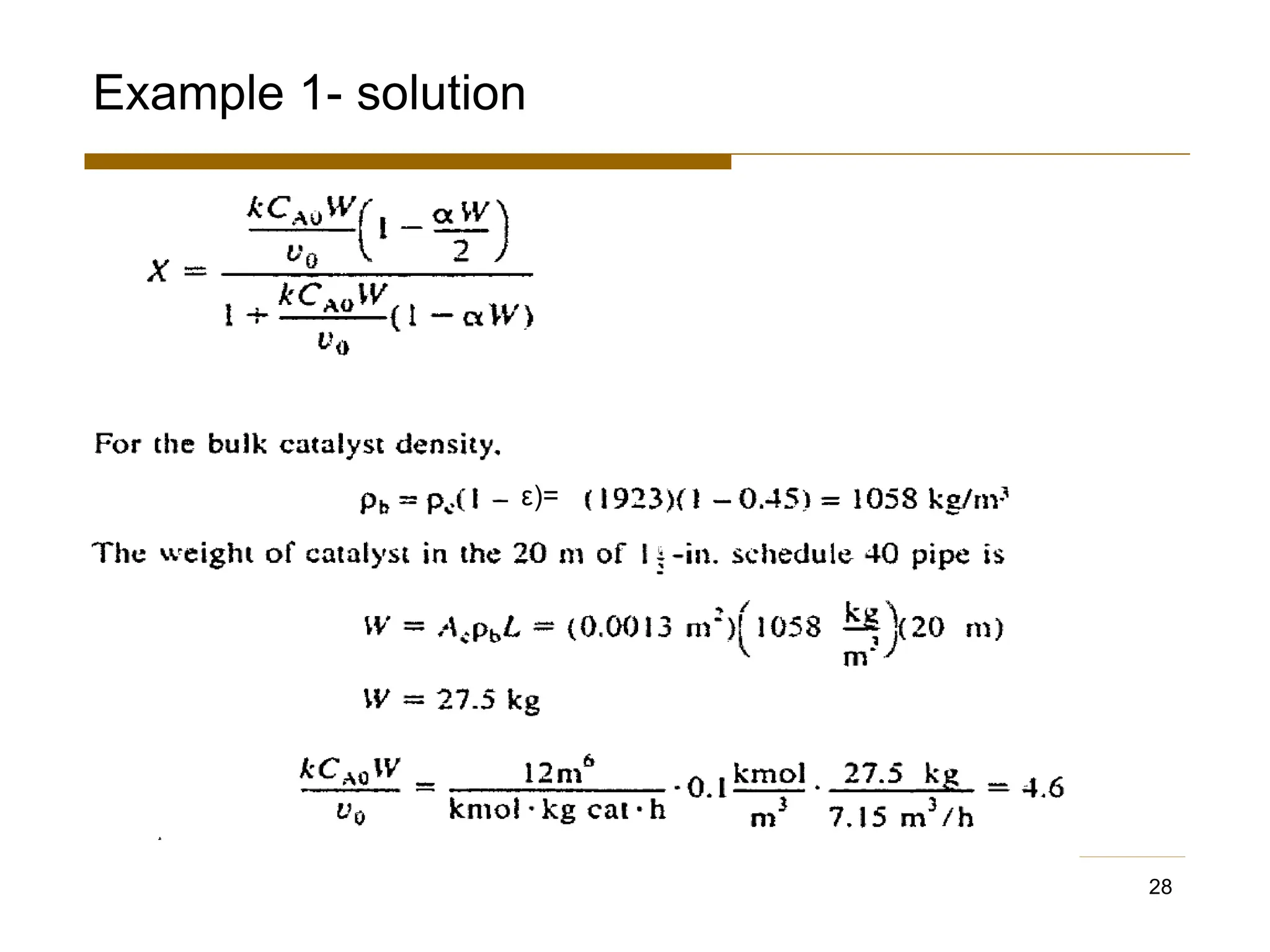
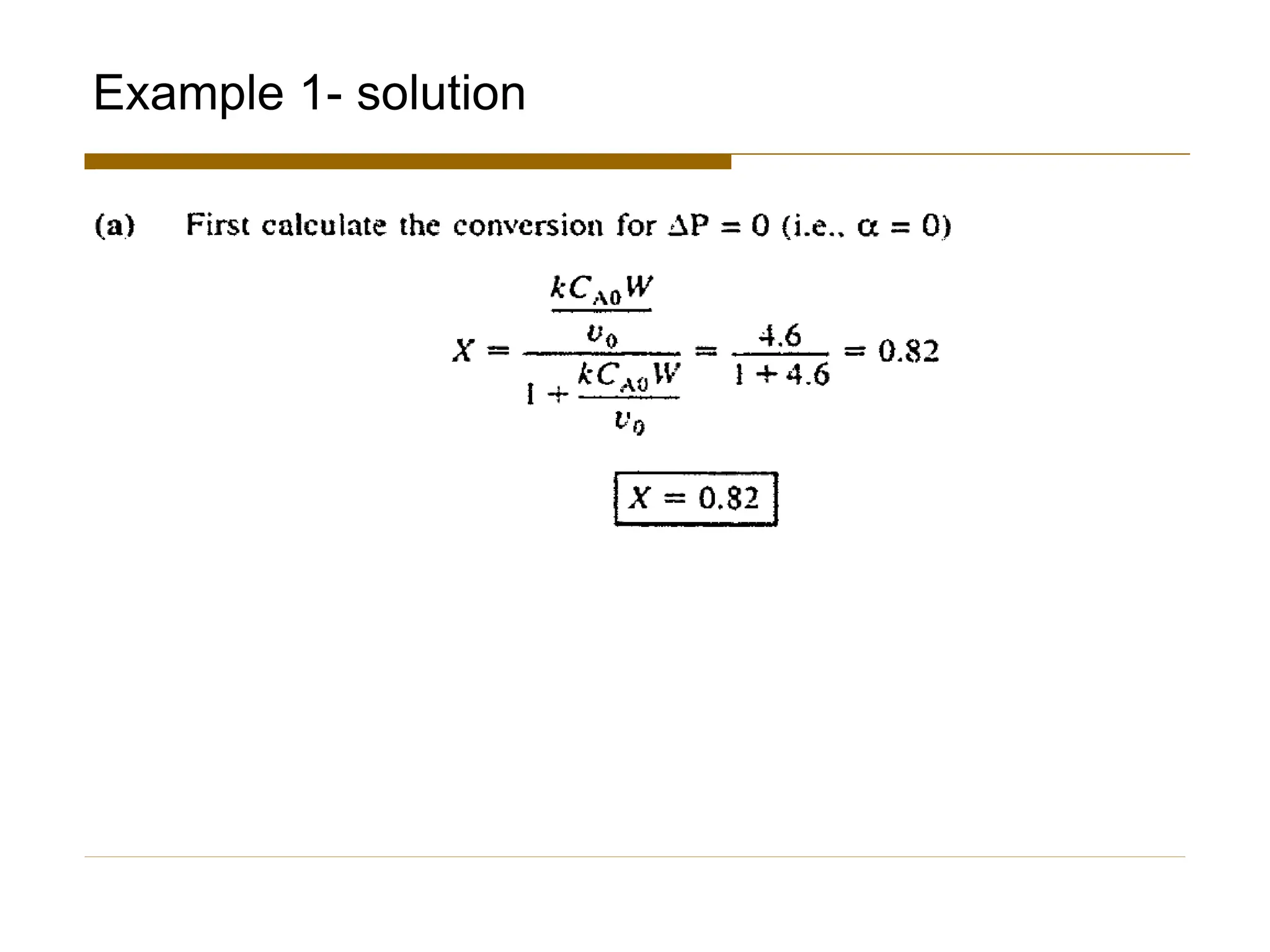
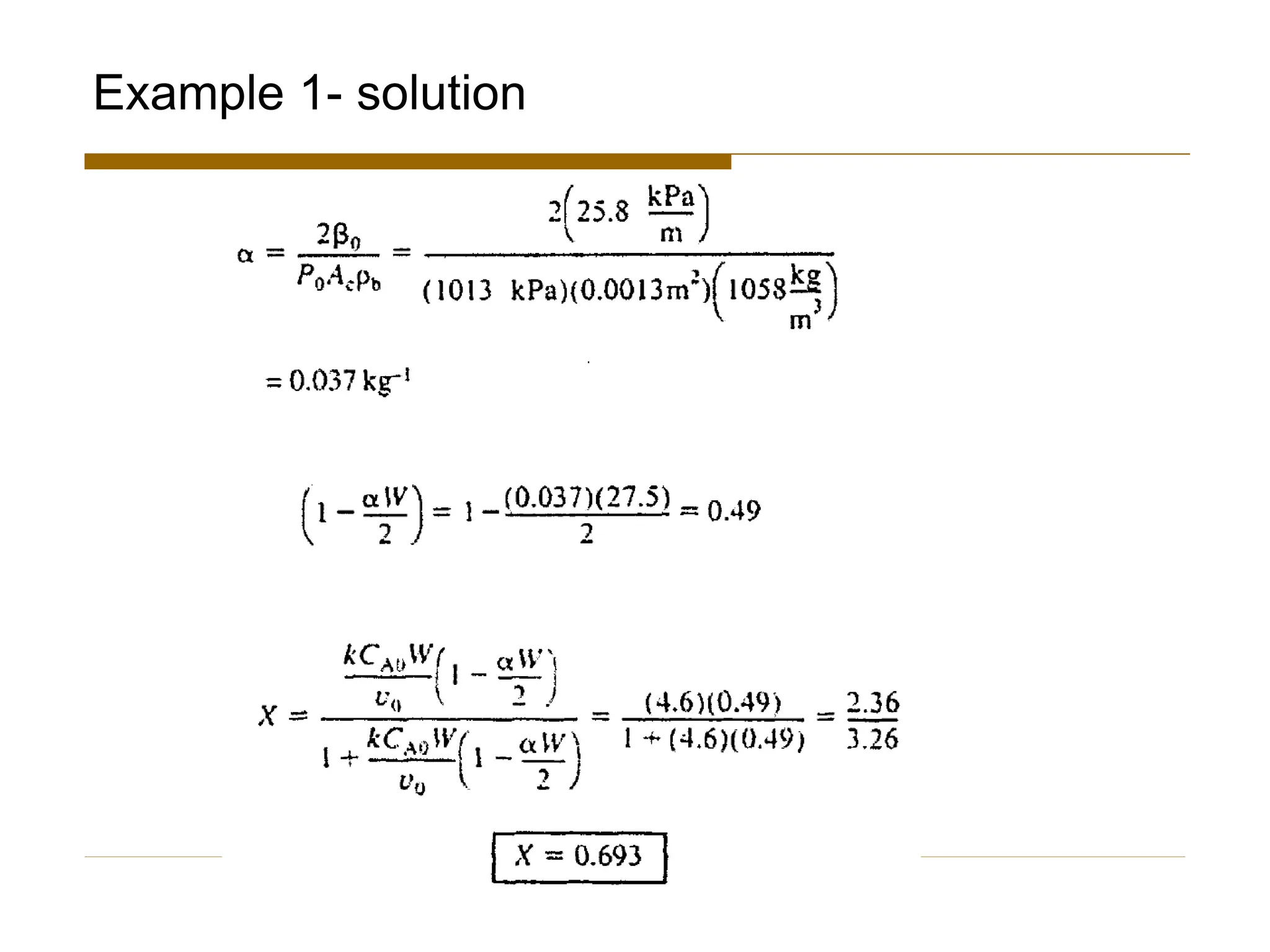
This document provides an overview of packed bed reactor design. It defines a packed bed reactor and lists learning outcomes which include determining catalyst weight, pressure drop parameters, and conversion. Advantages and disadvantages of packed bed reactors are given. Equations for governing reactor sizing are presented, including equations relating volumetric reaction rate, catalyst weight, and pressure drop. The impact of pressure drop on gas versus liquid phase reactions is discussed. The Ergun equation for calculating pressure drop is derived. Finally, an example problem is given to demonstrate the effect of pressure drop on conversion.