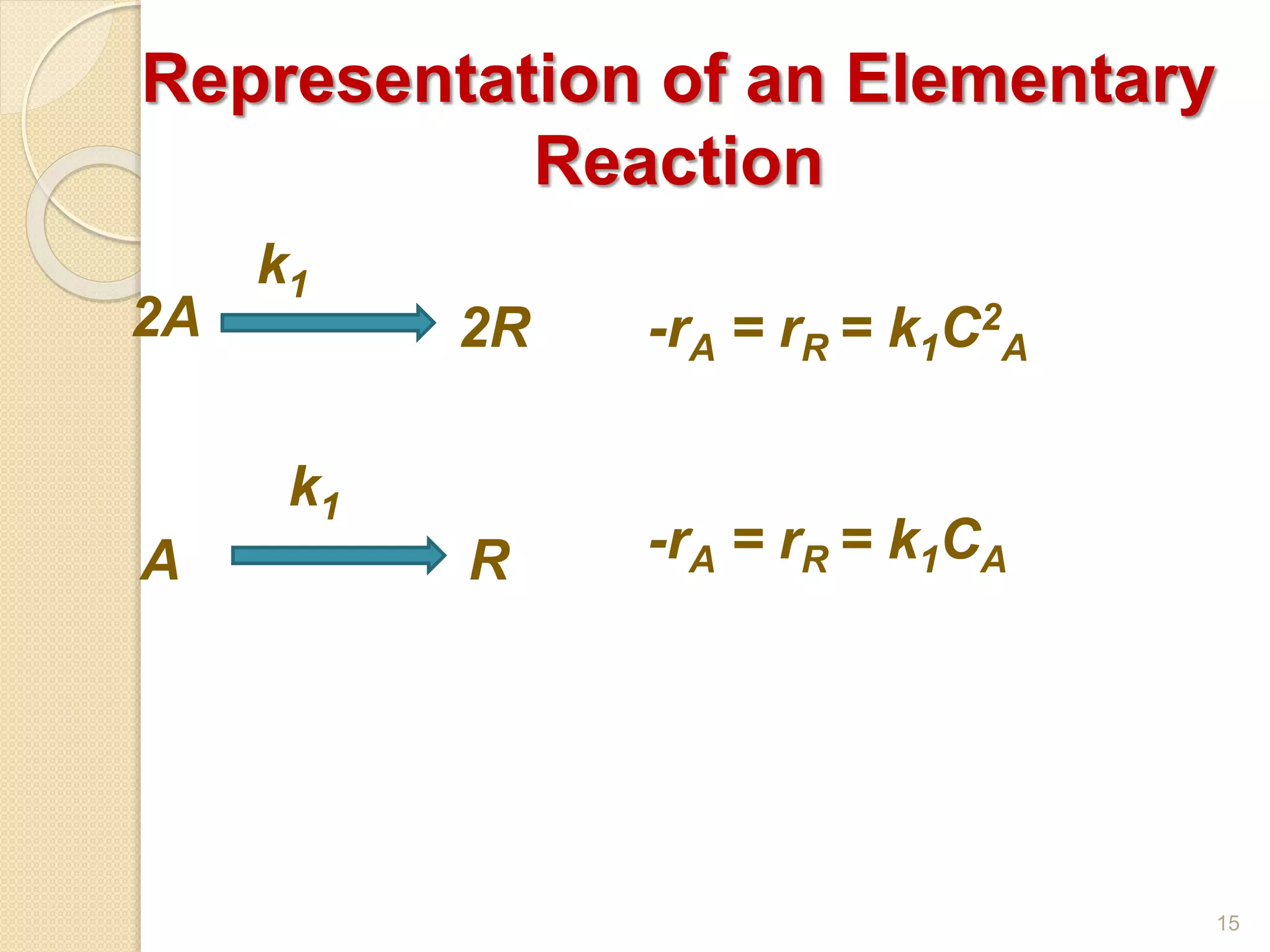
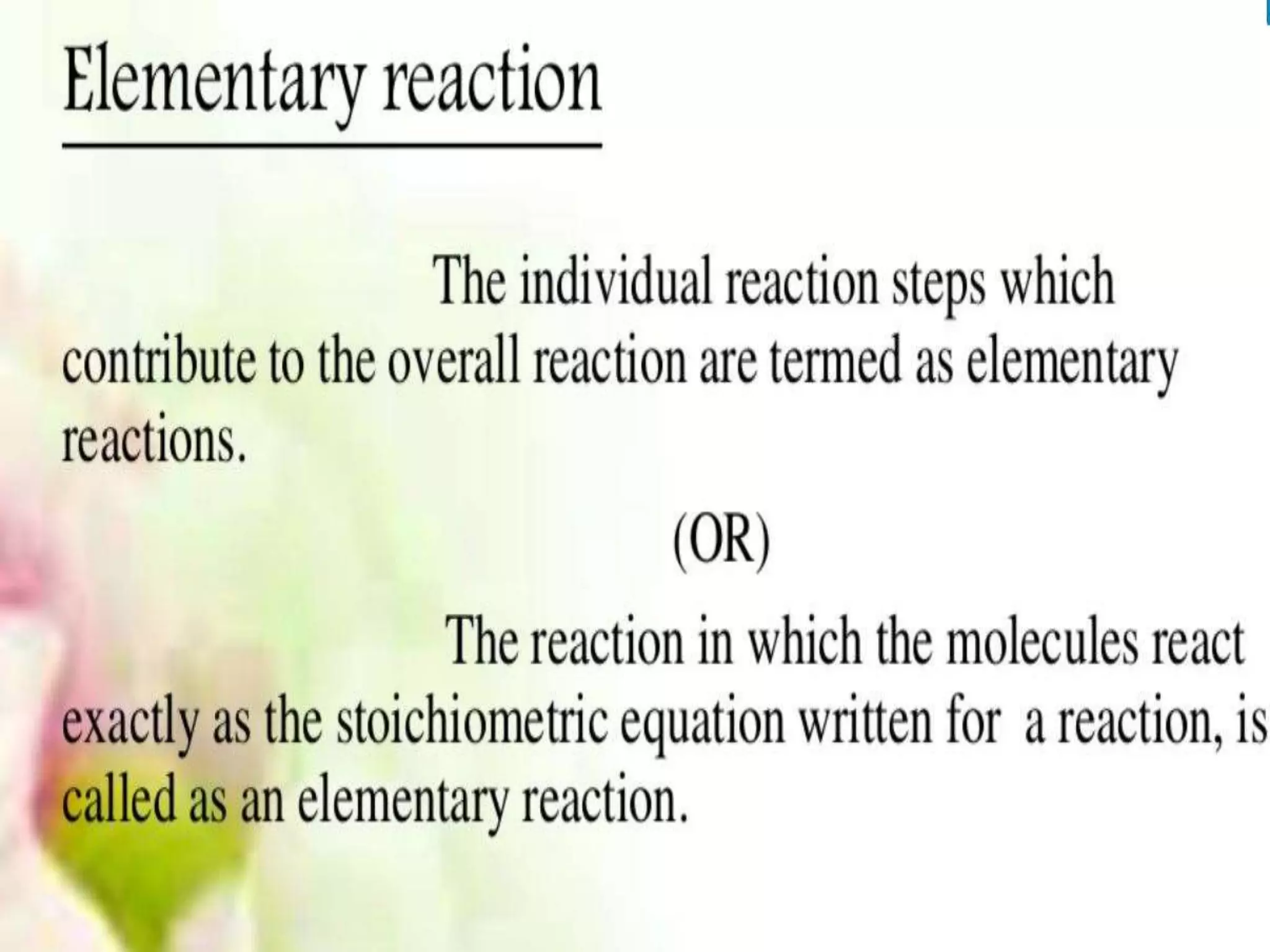
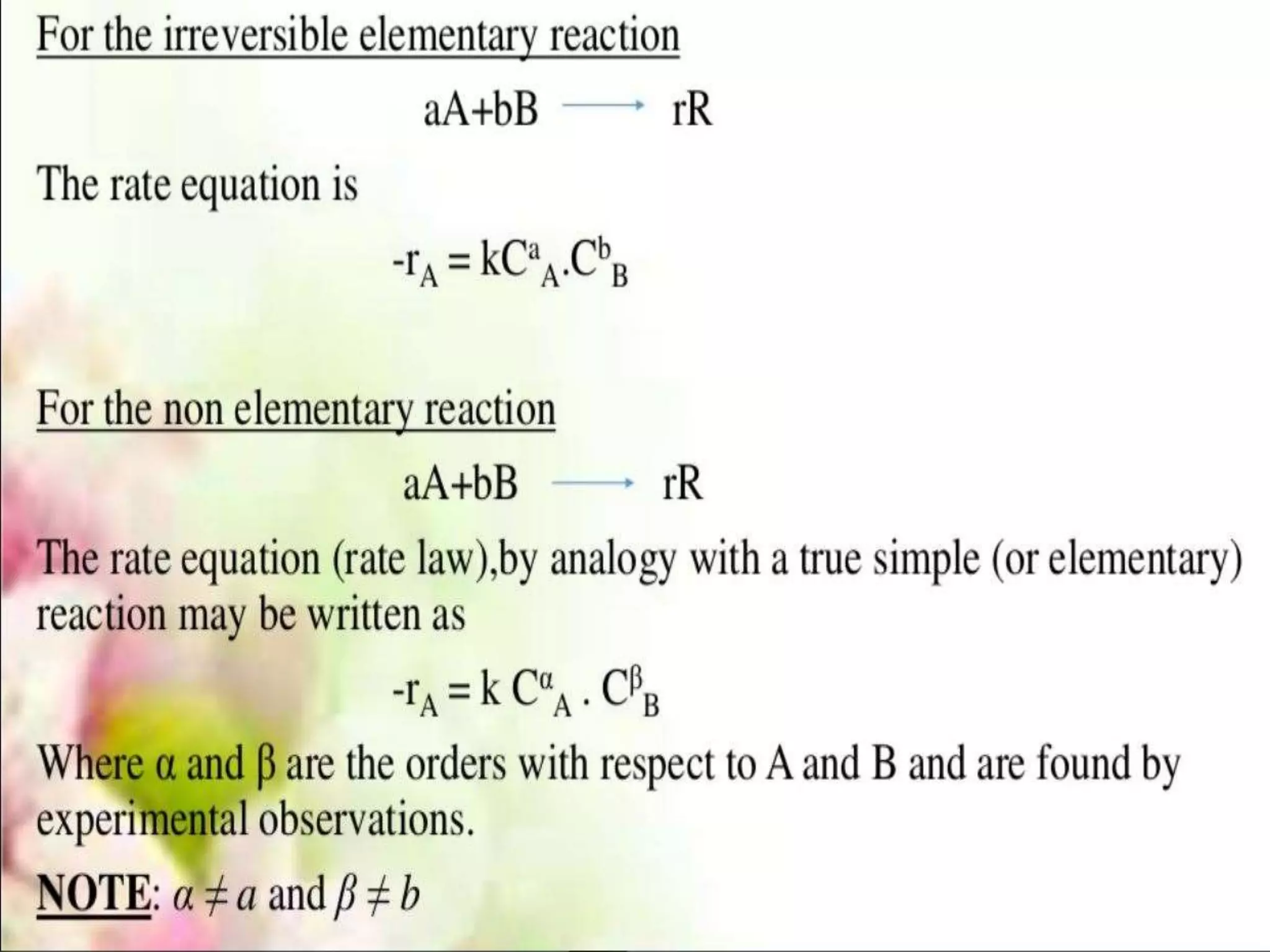
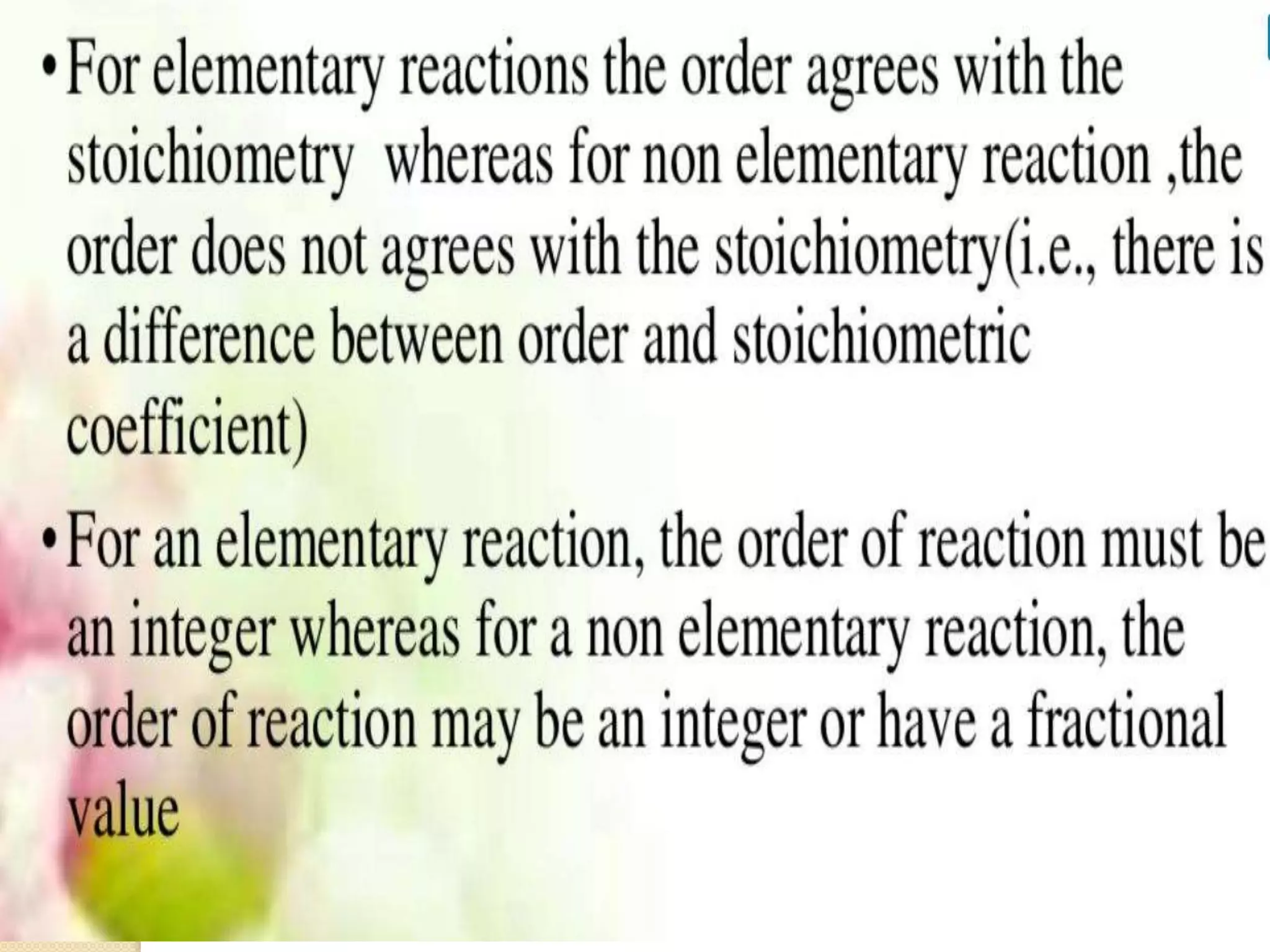
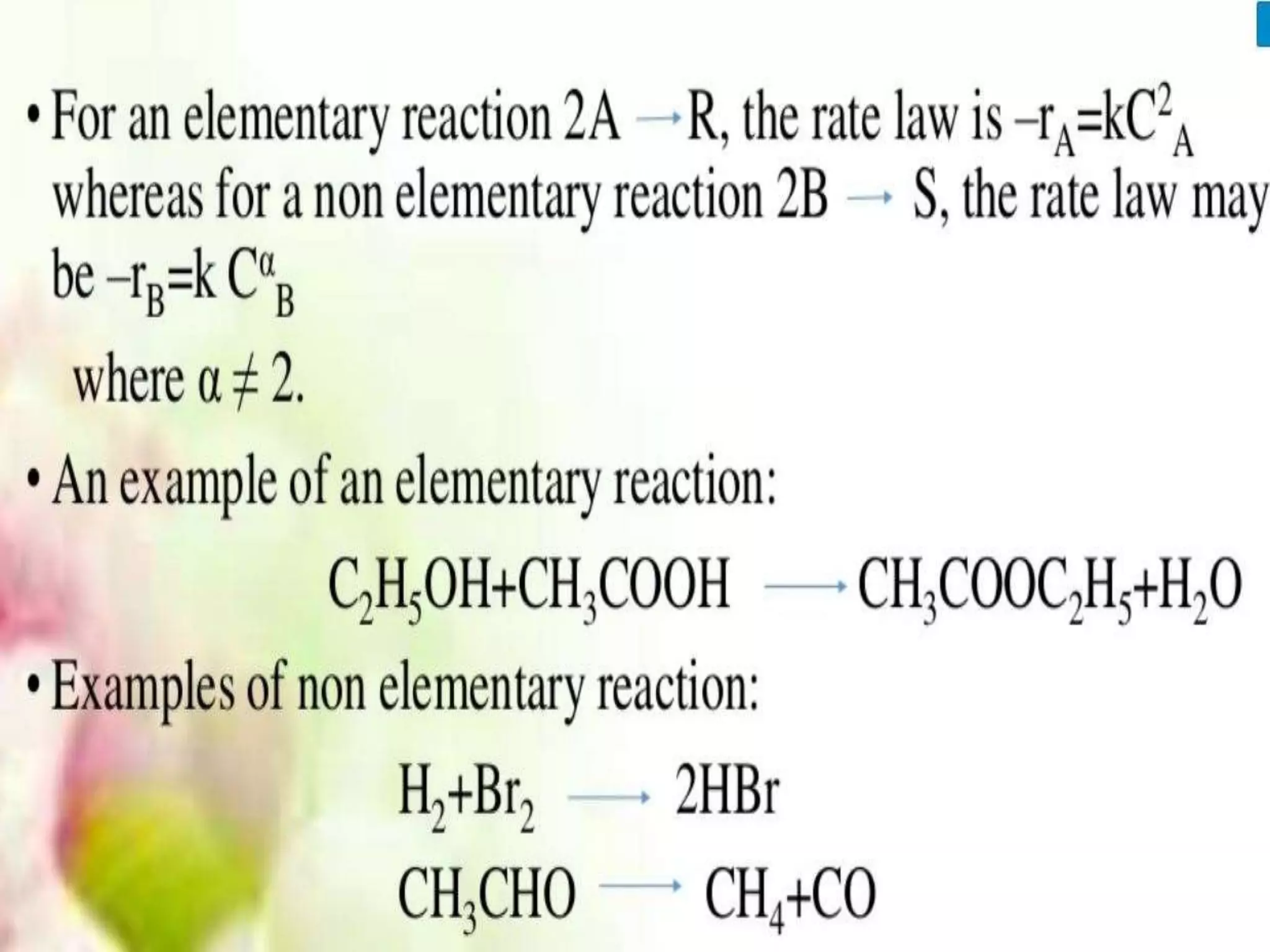
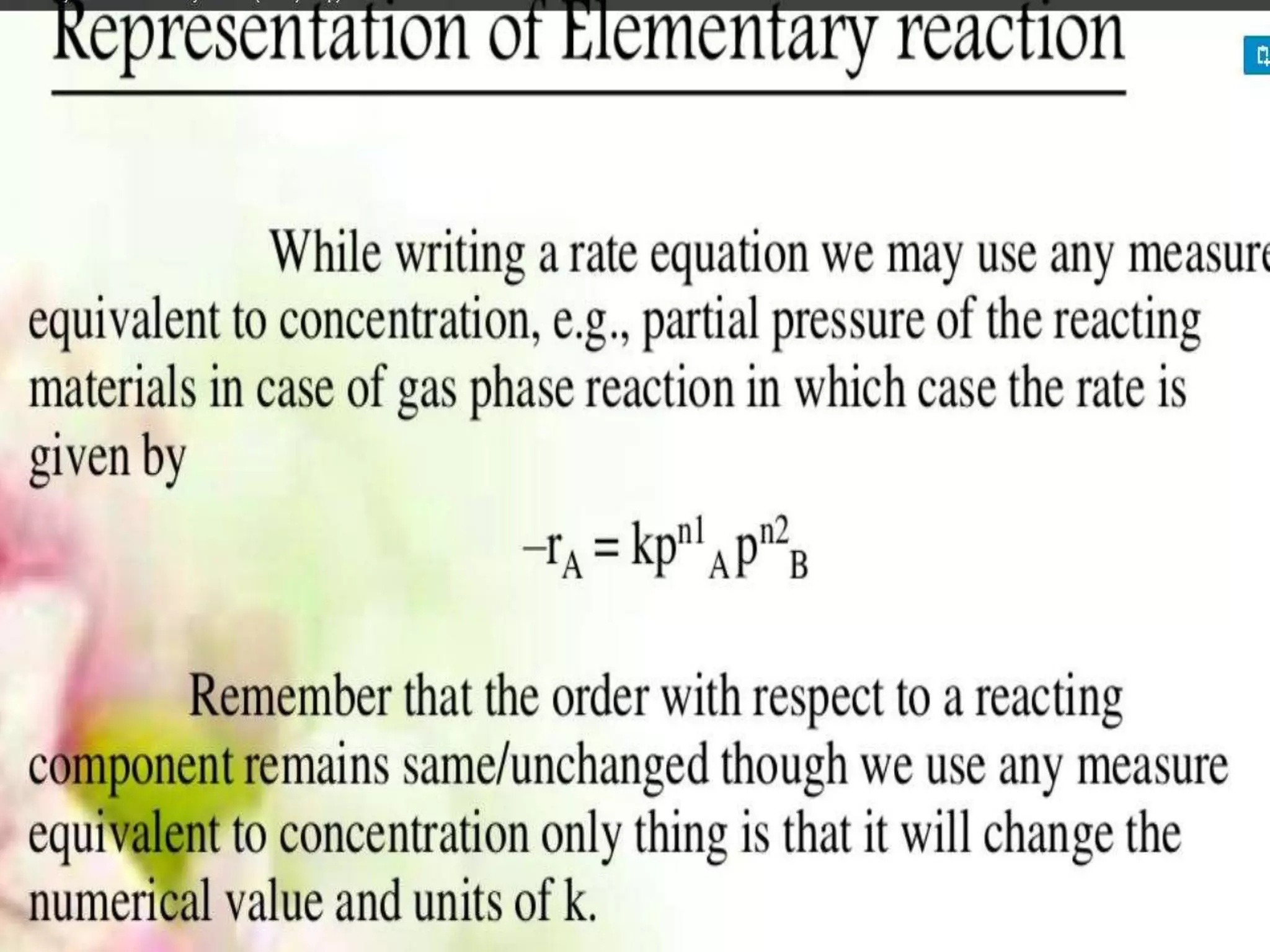
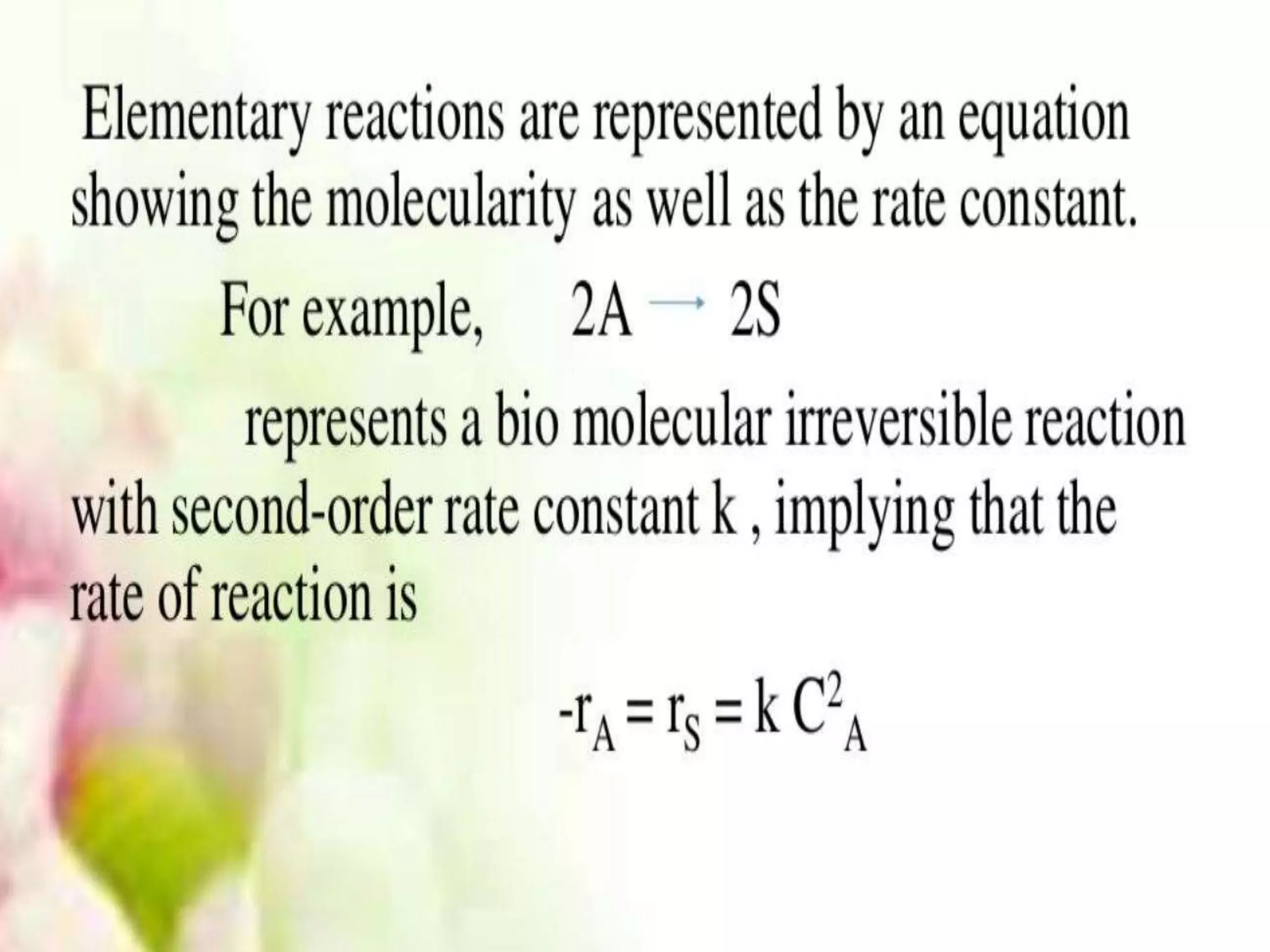
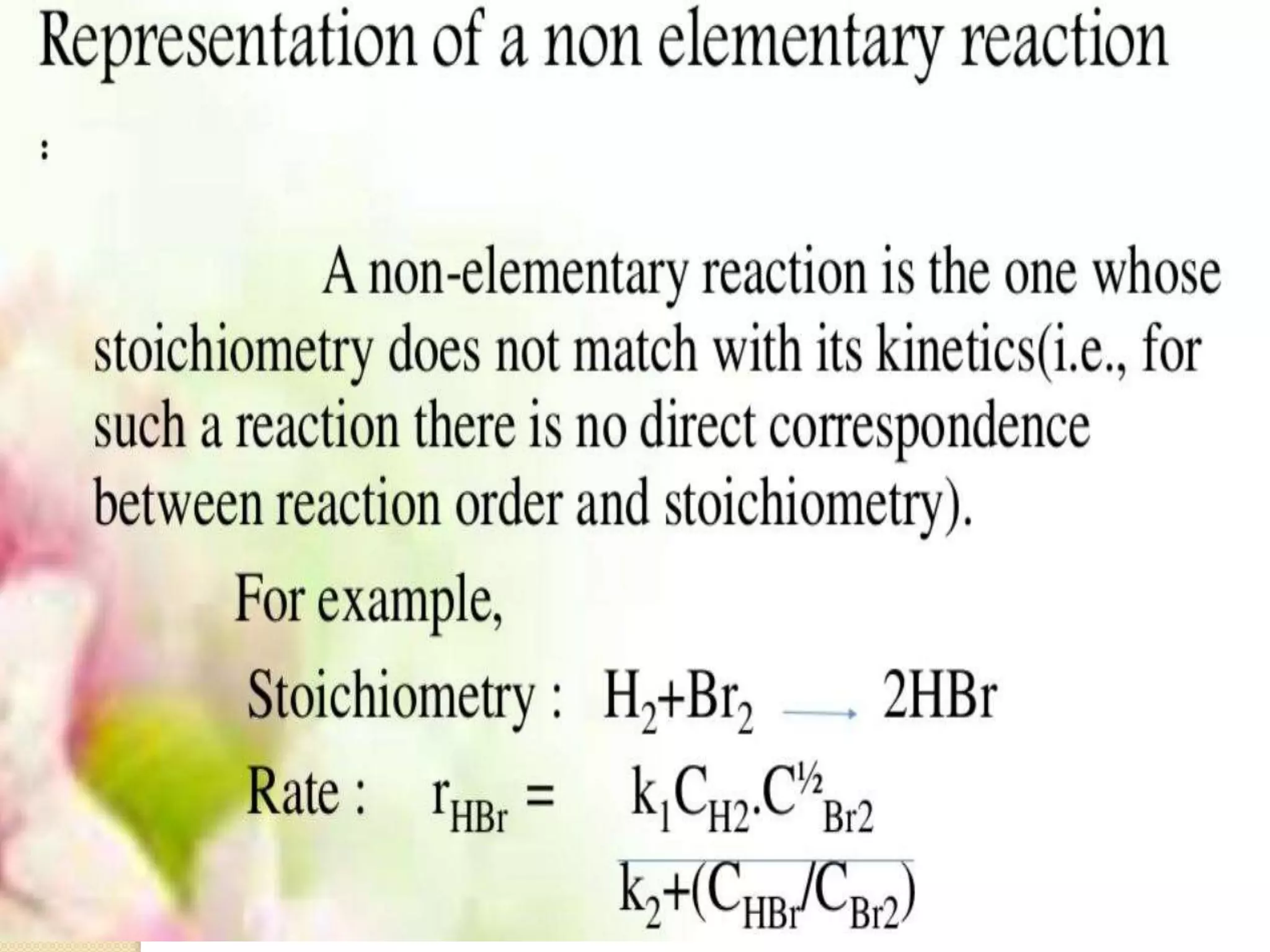
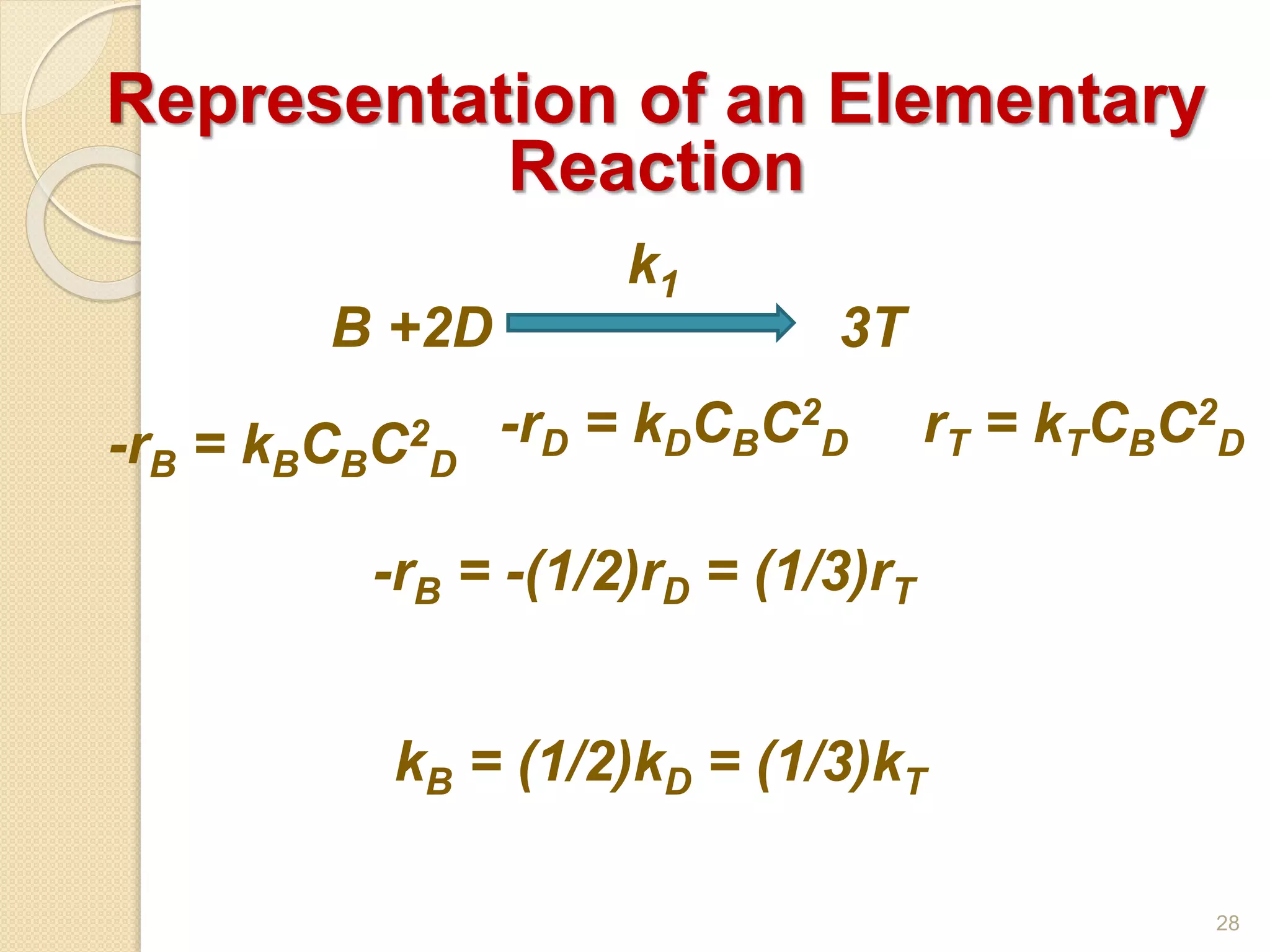
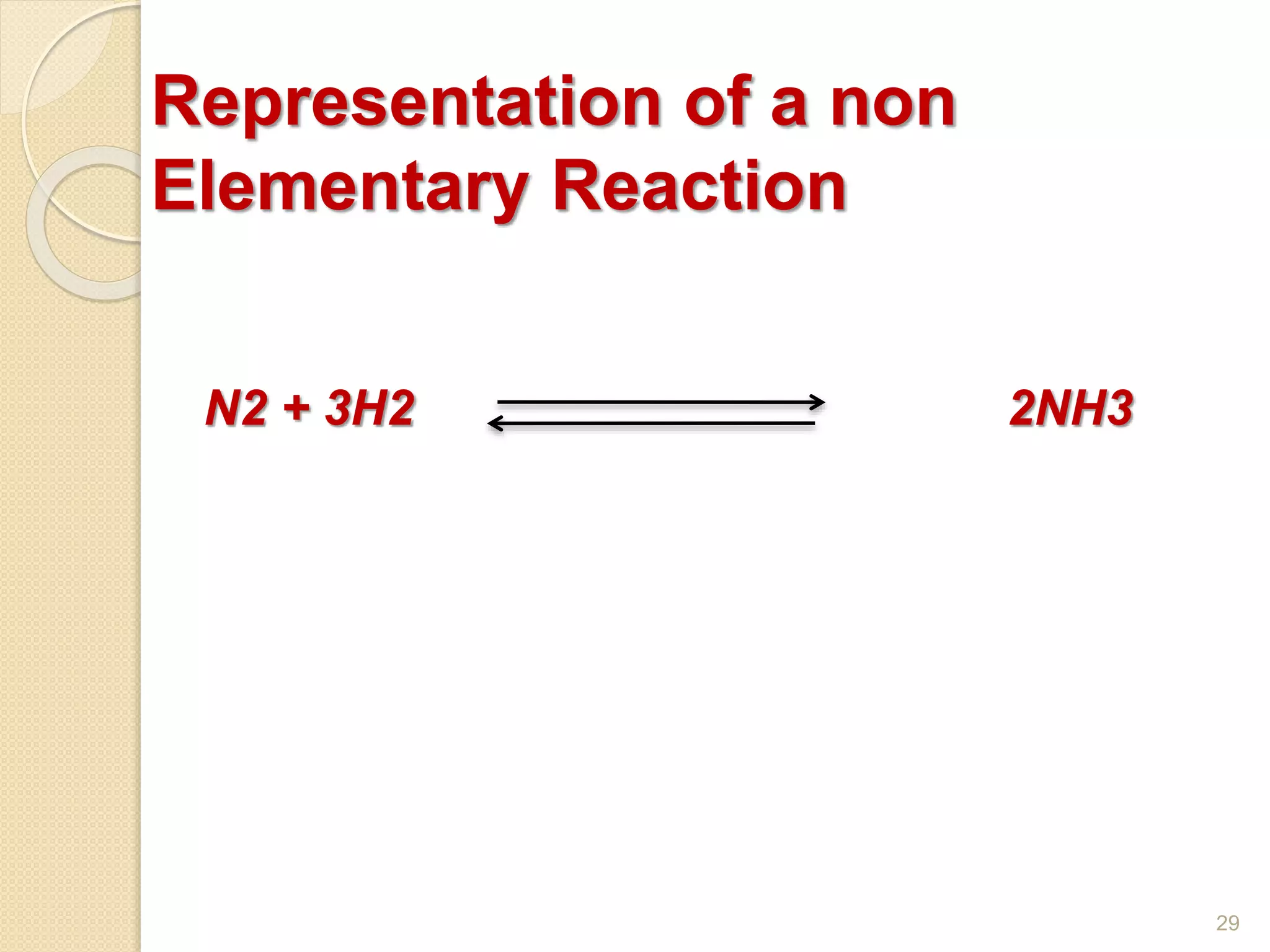
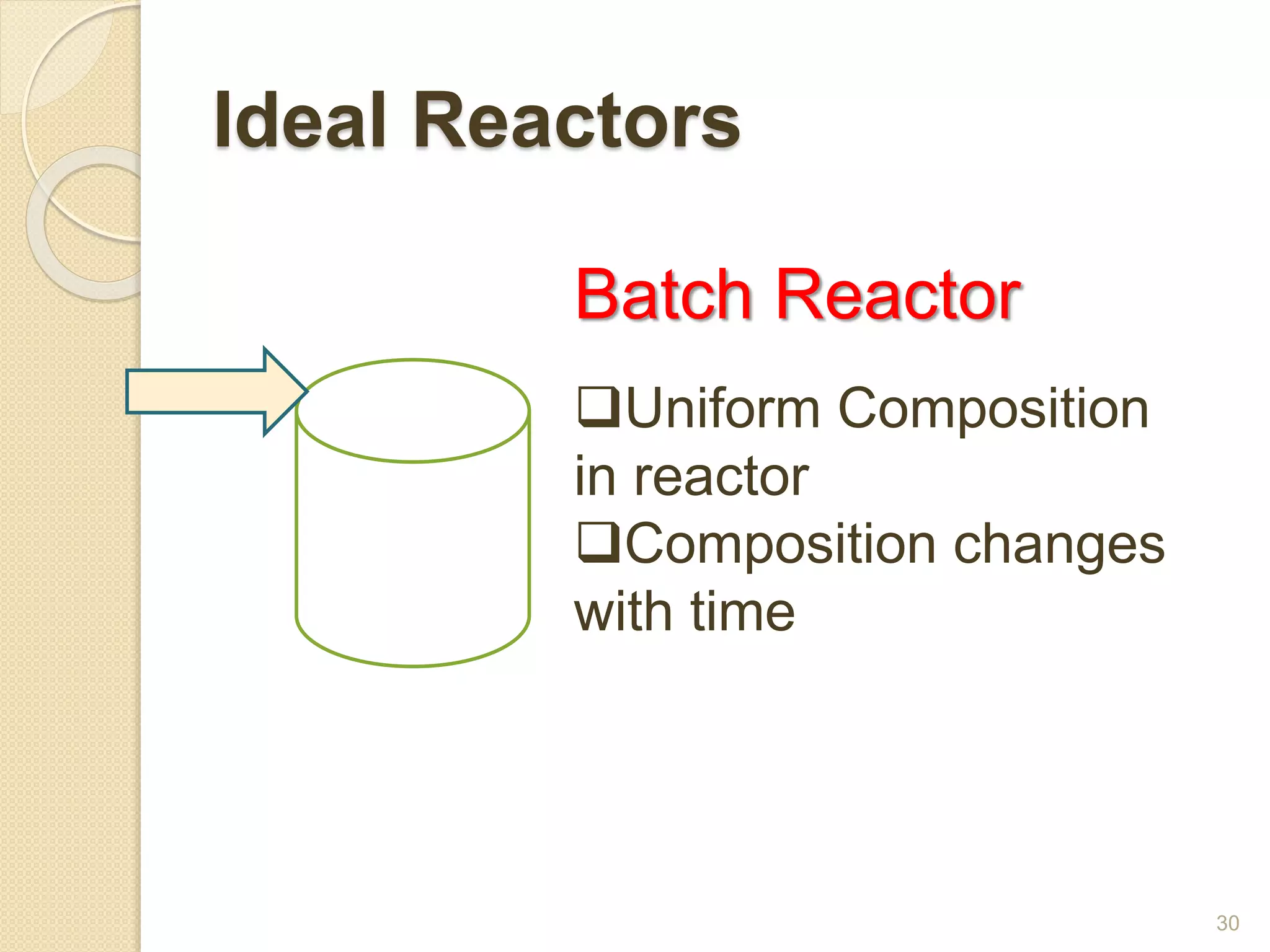
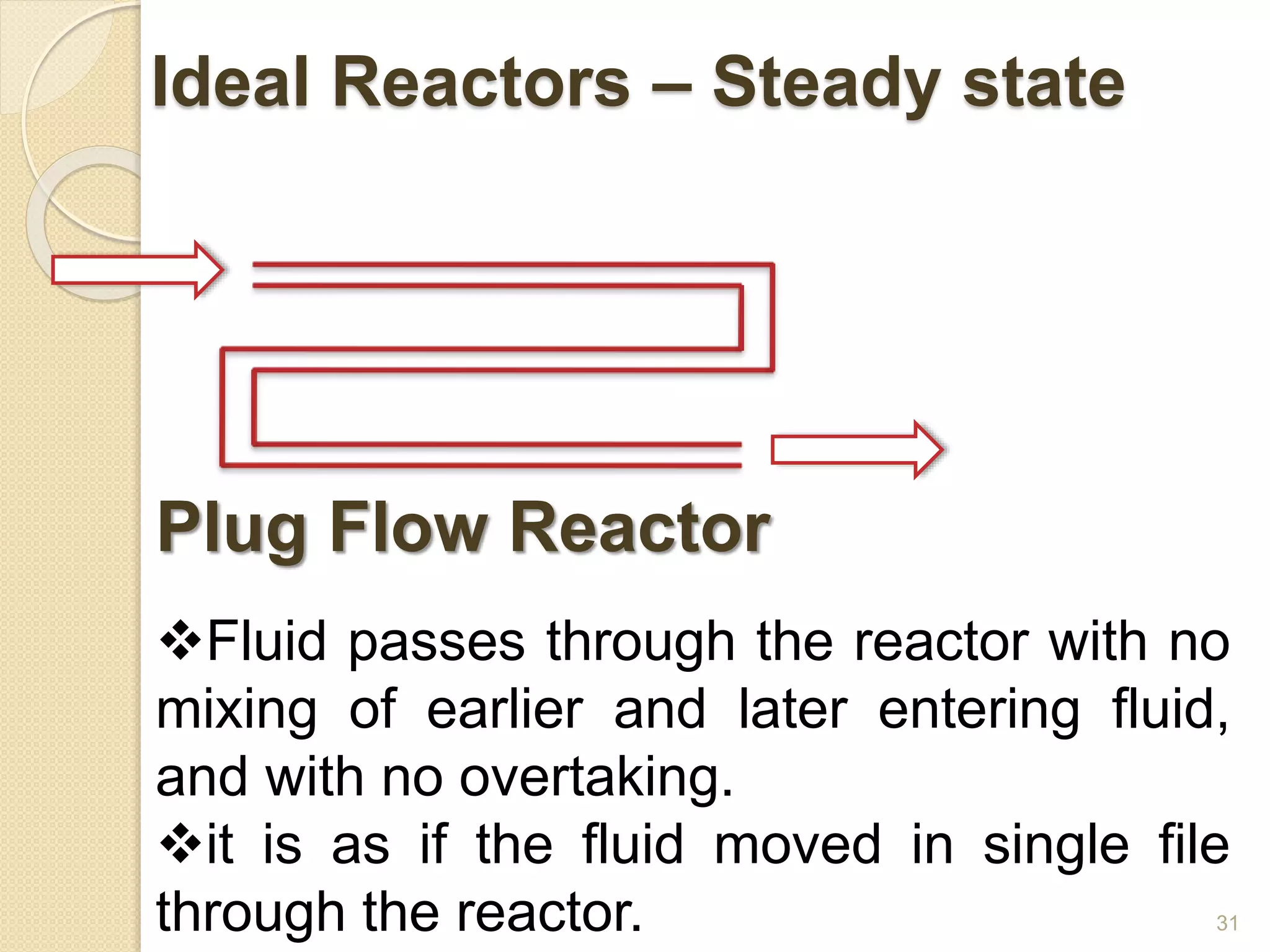
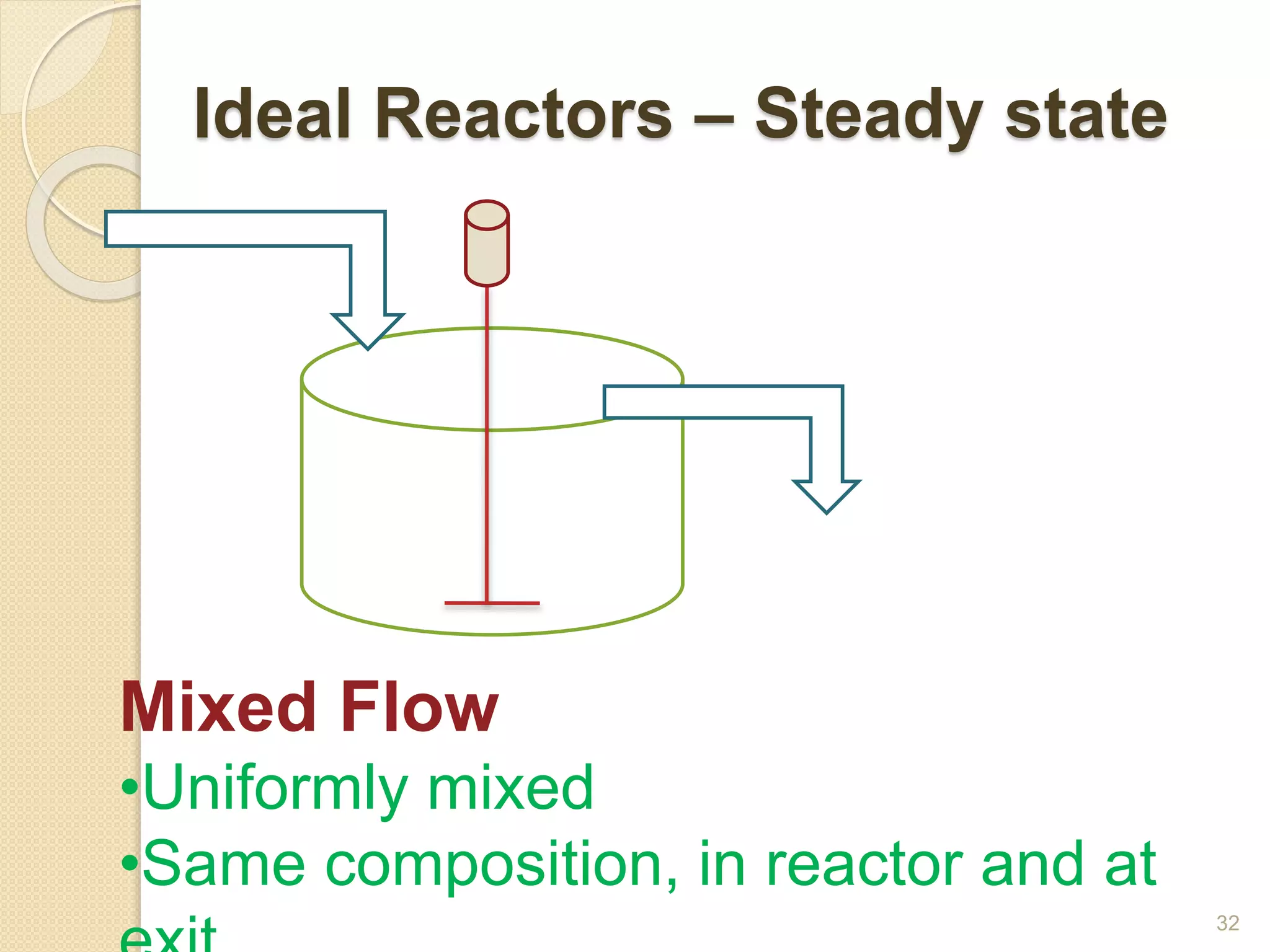
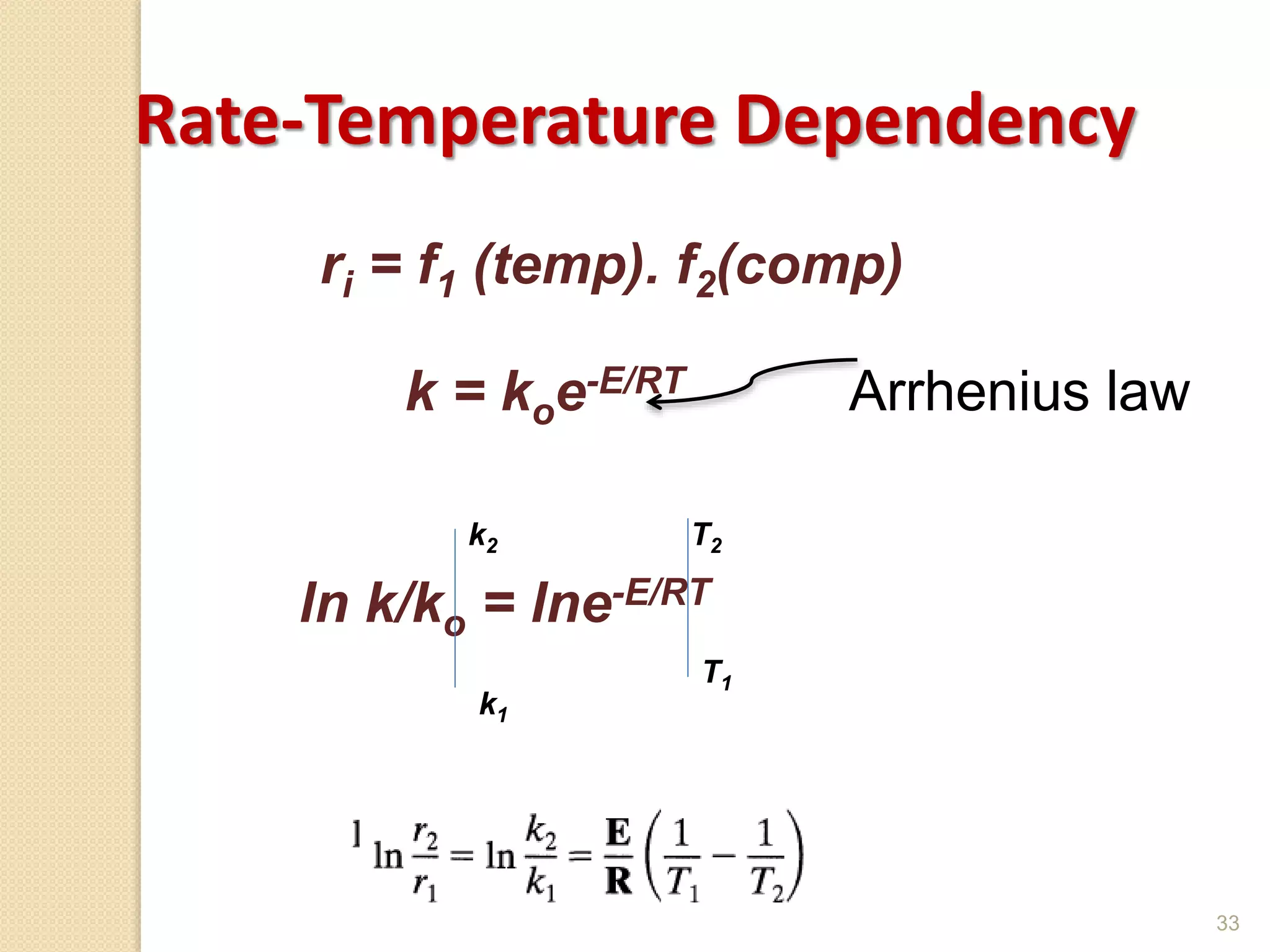
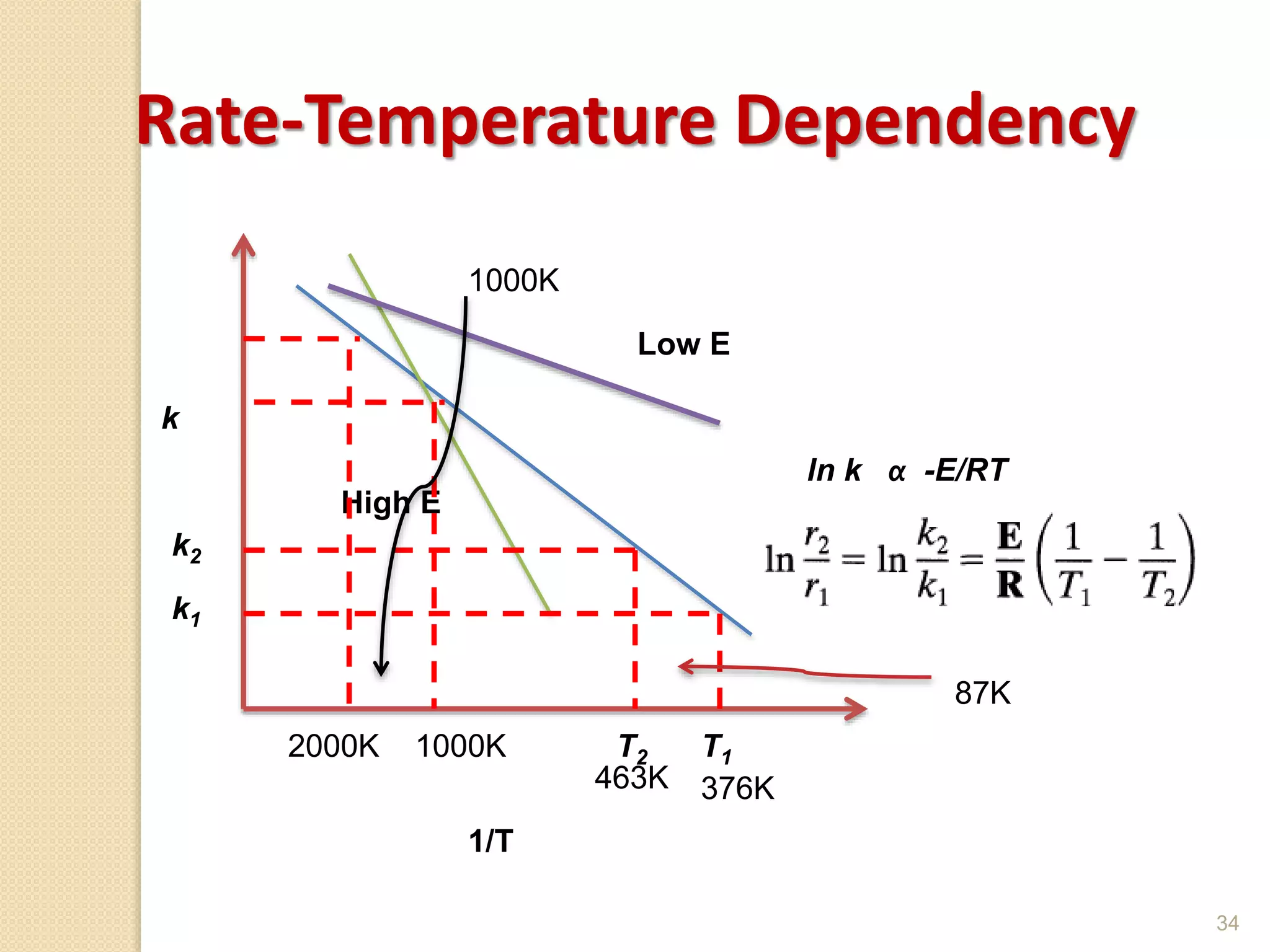
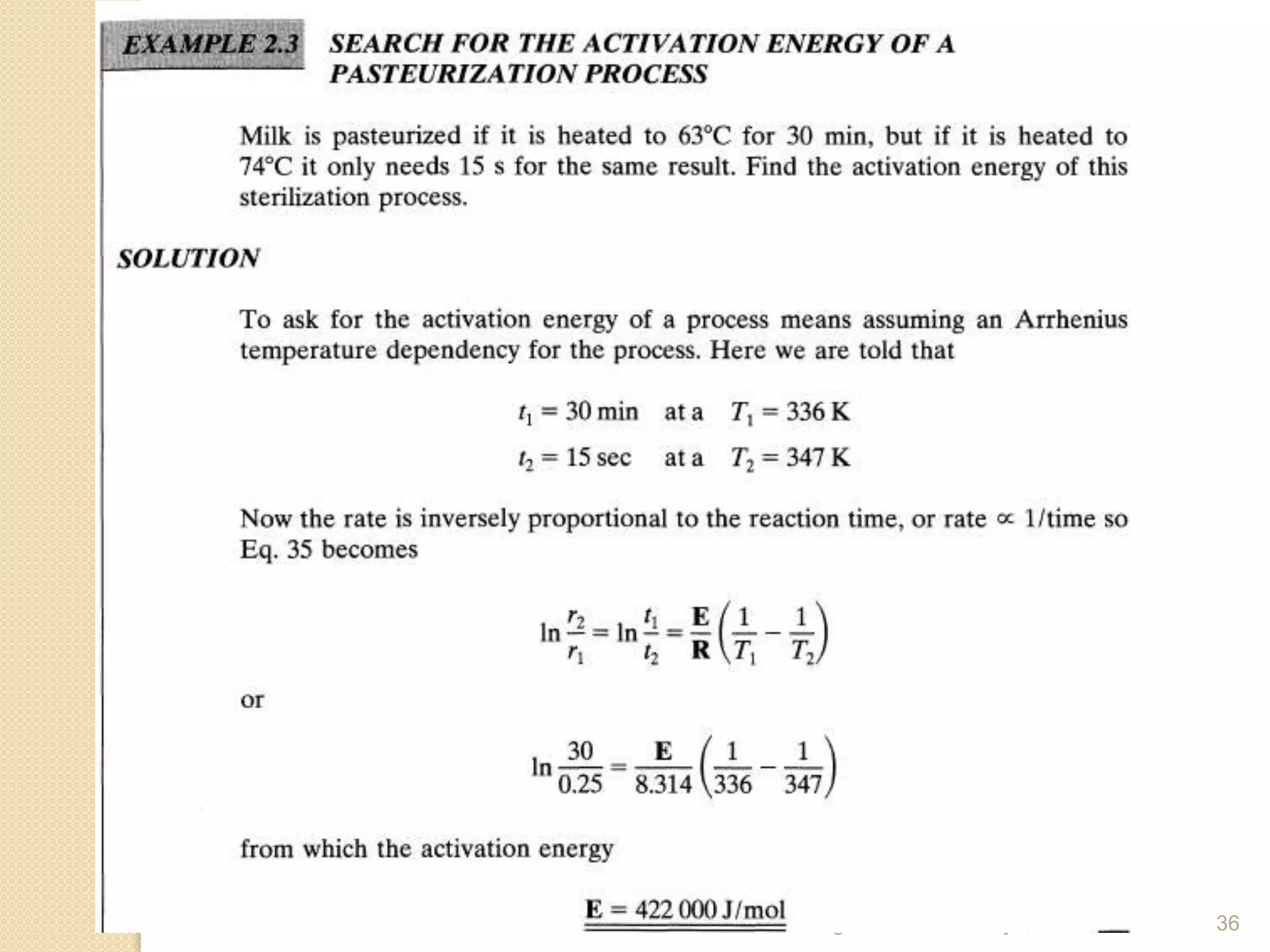
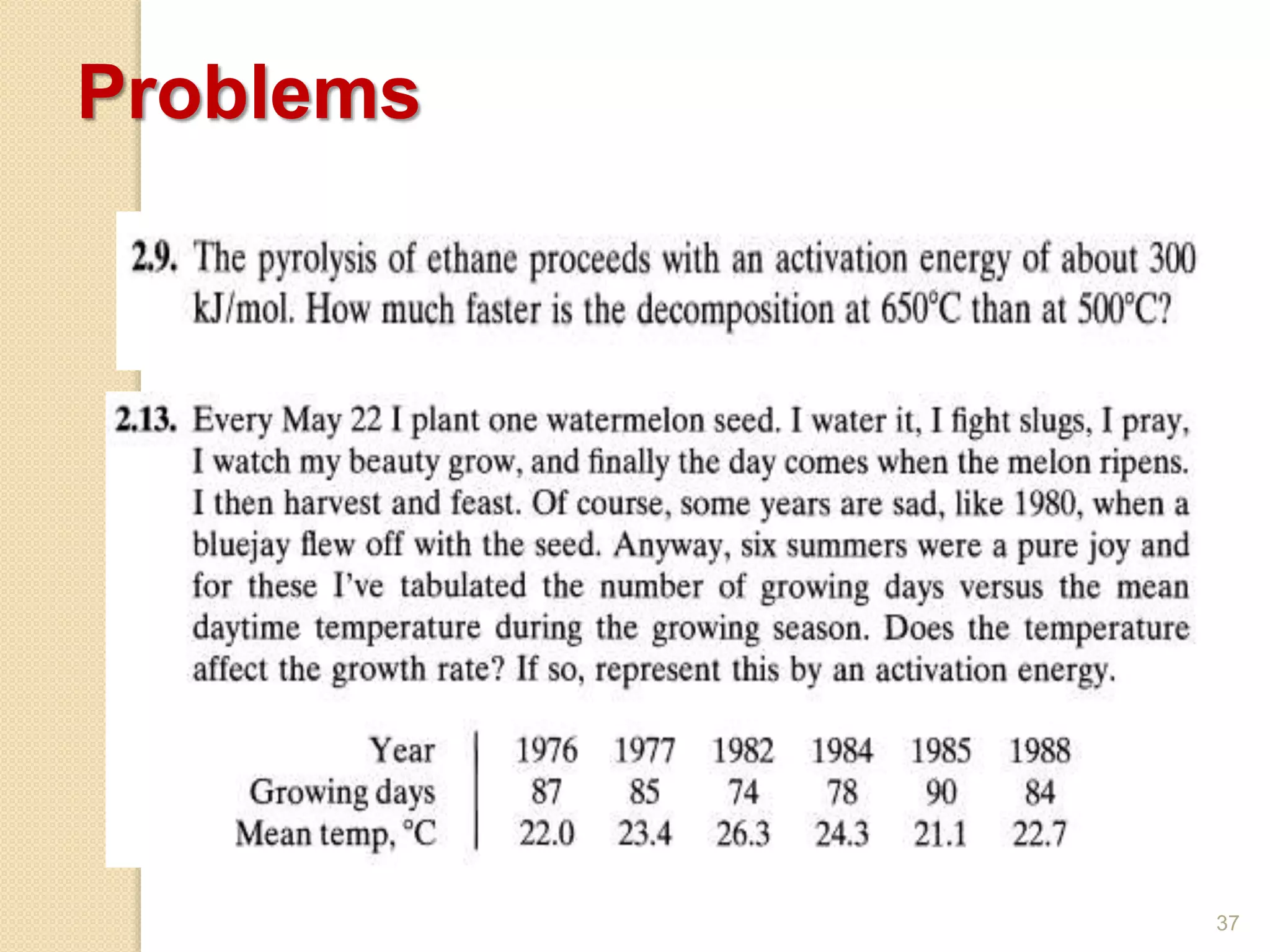
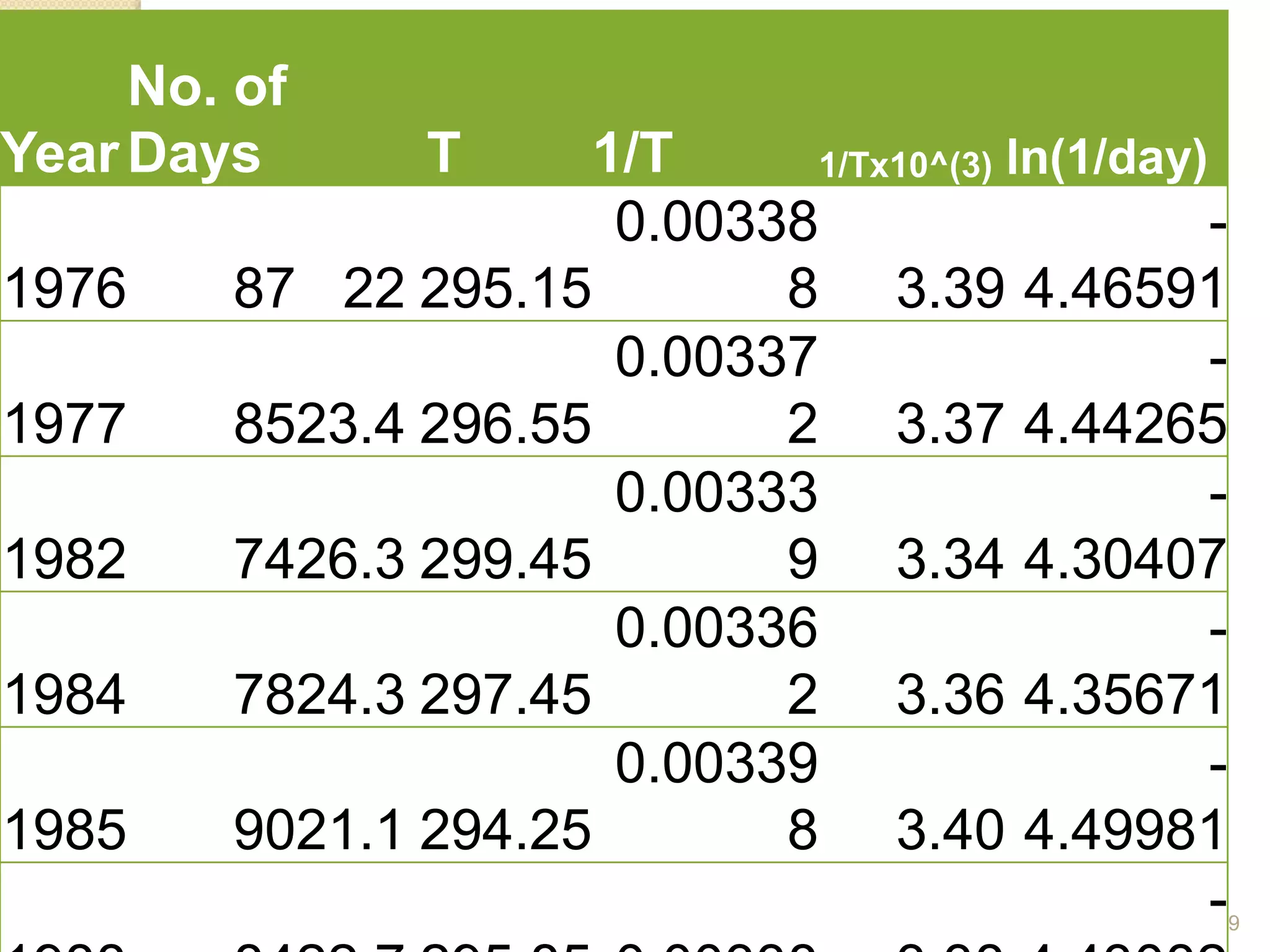
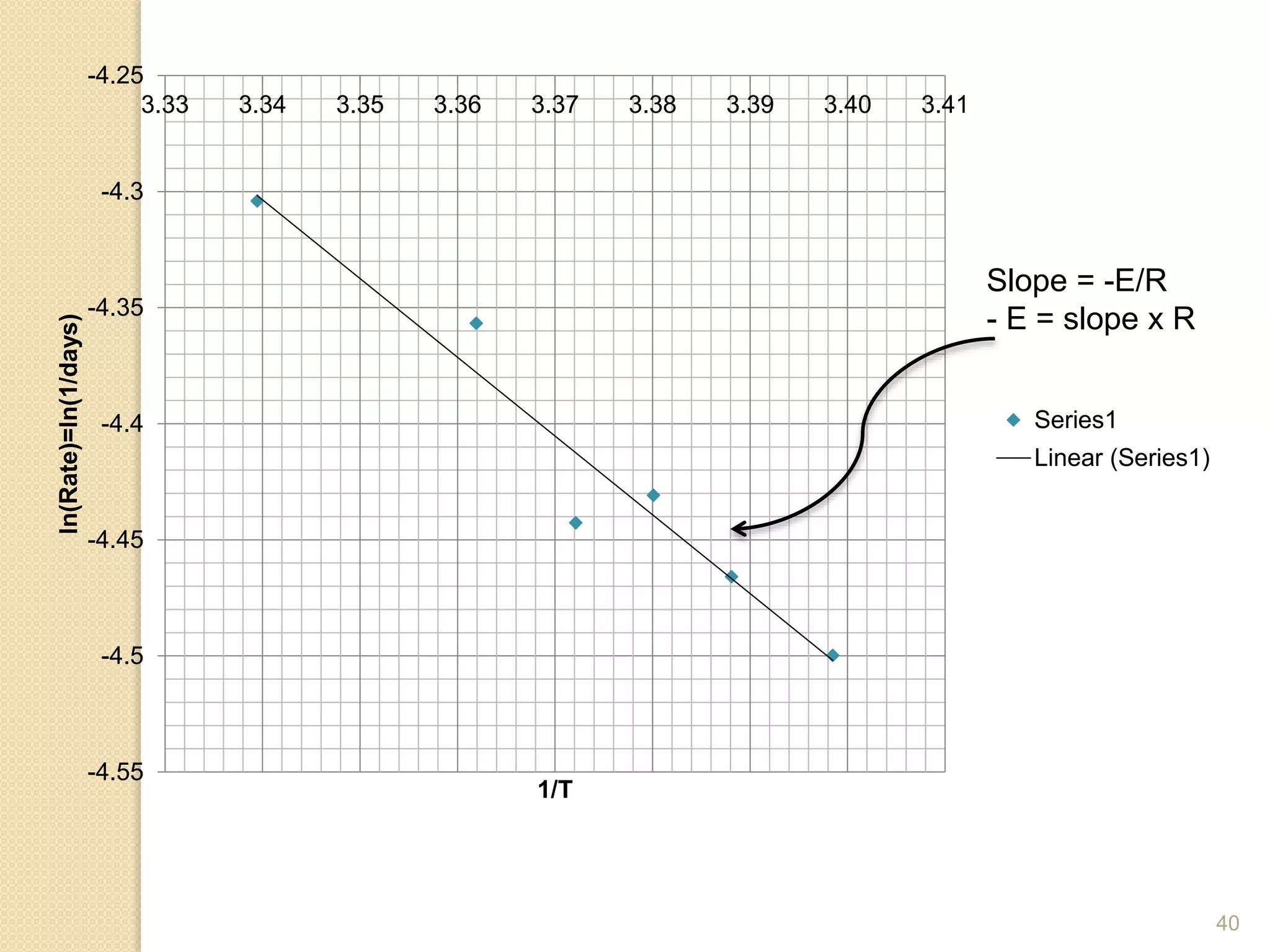
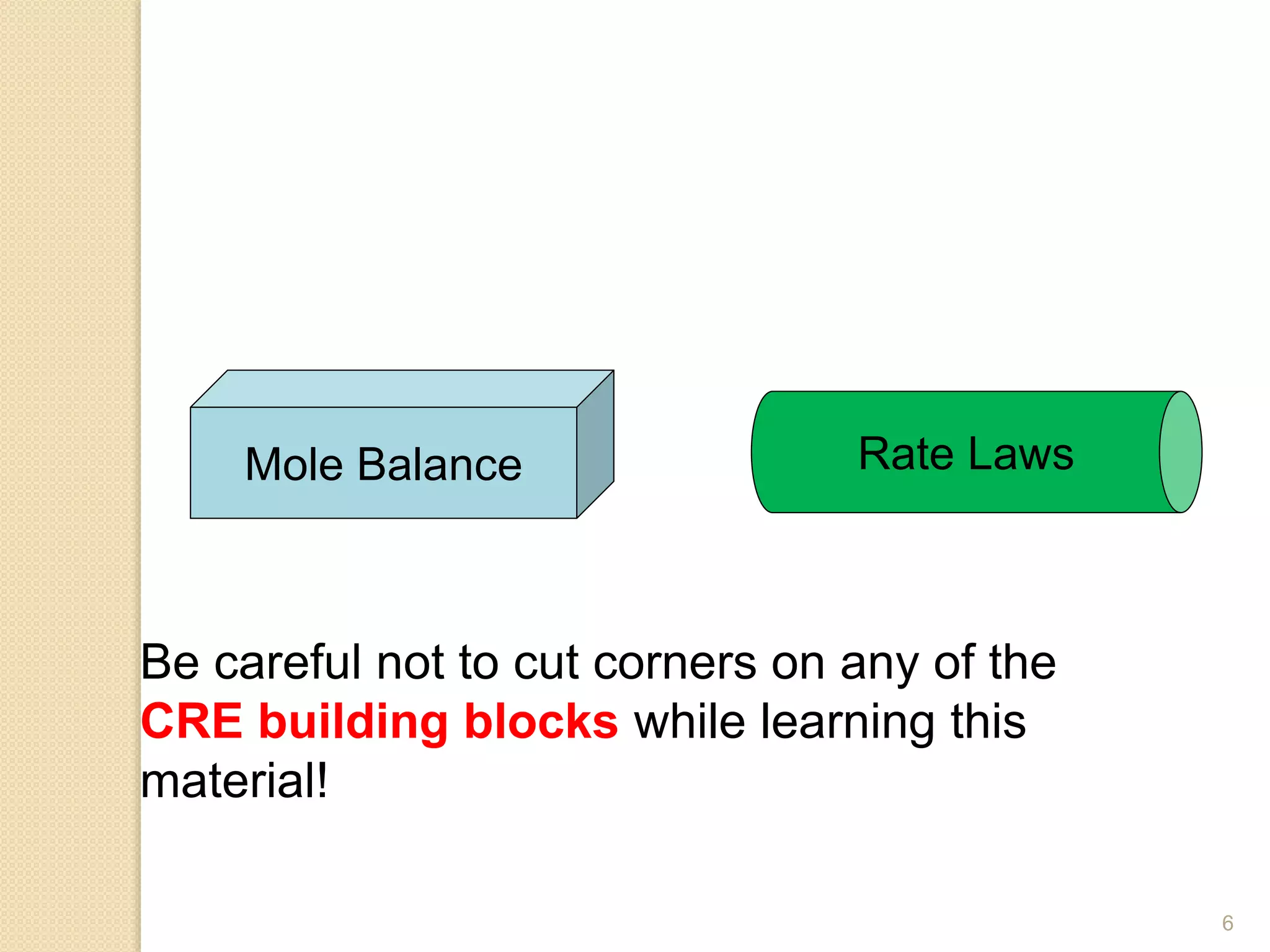
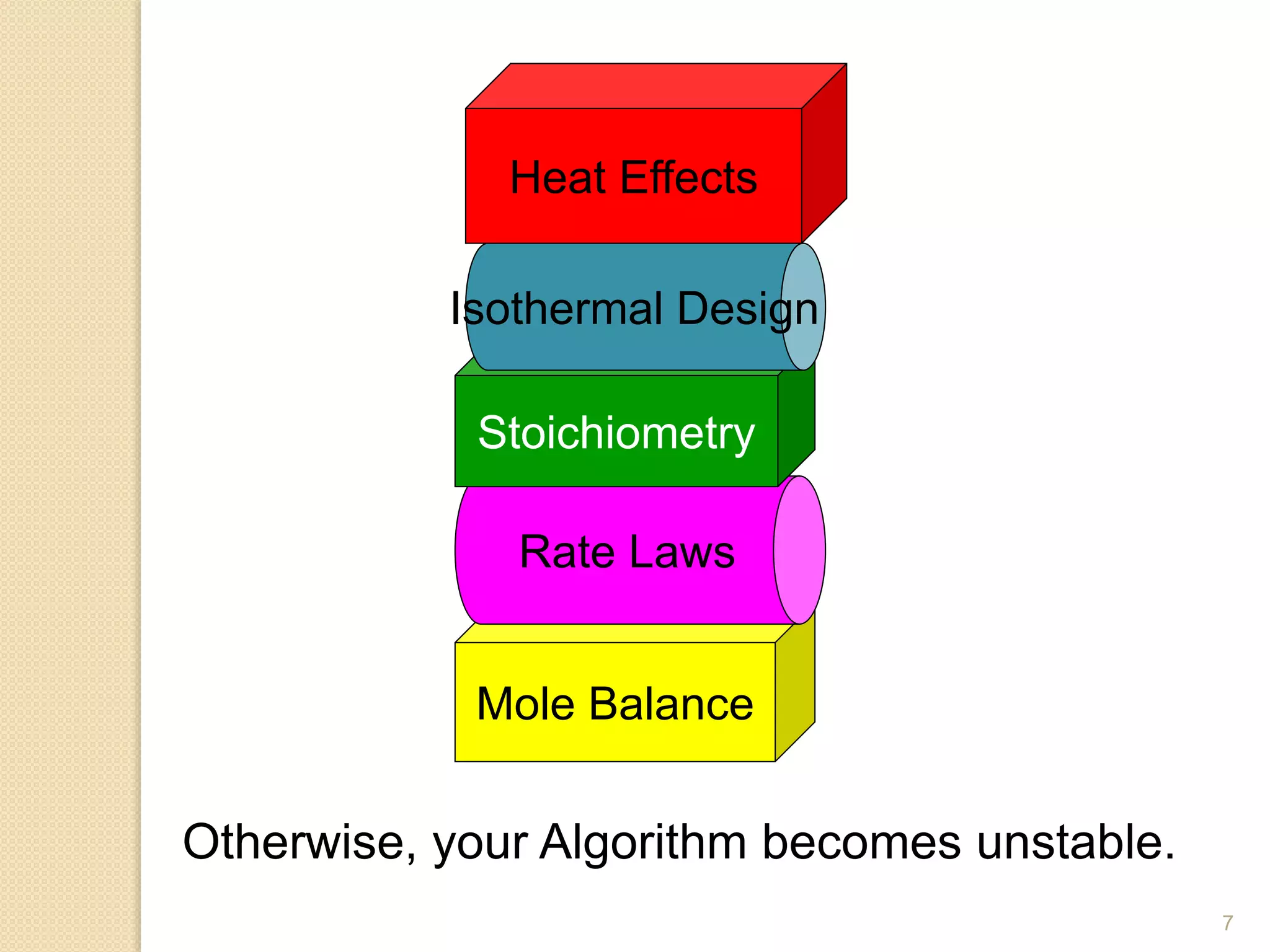
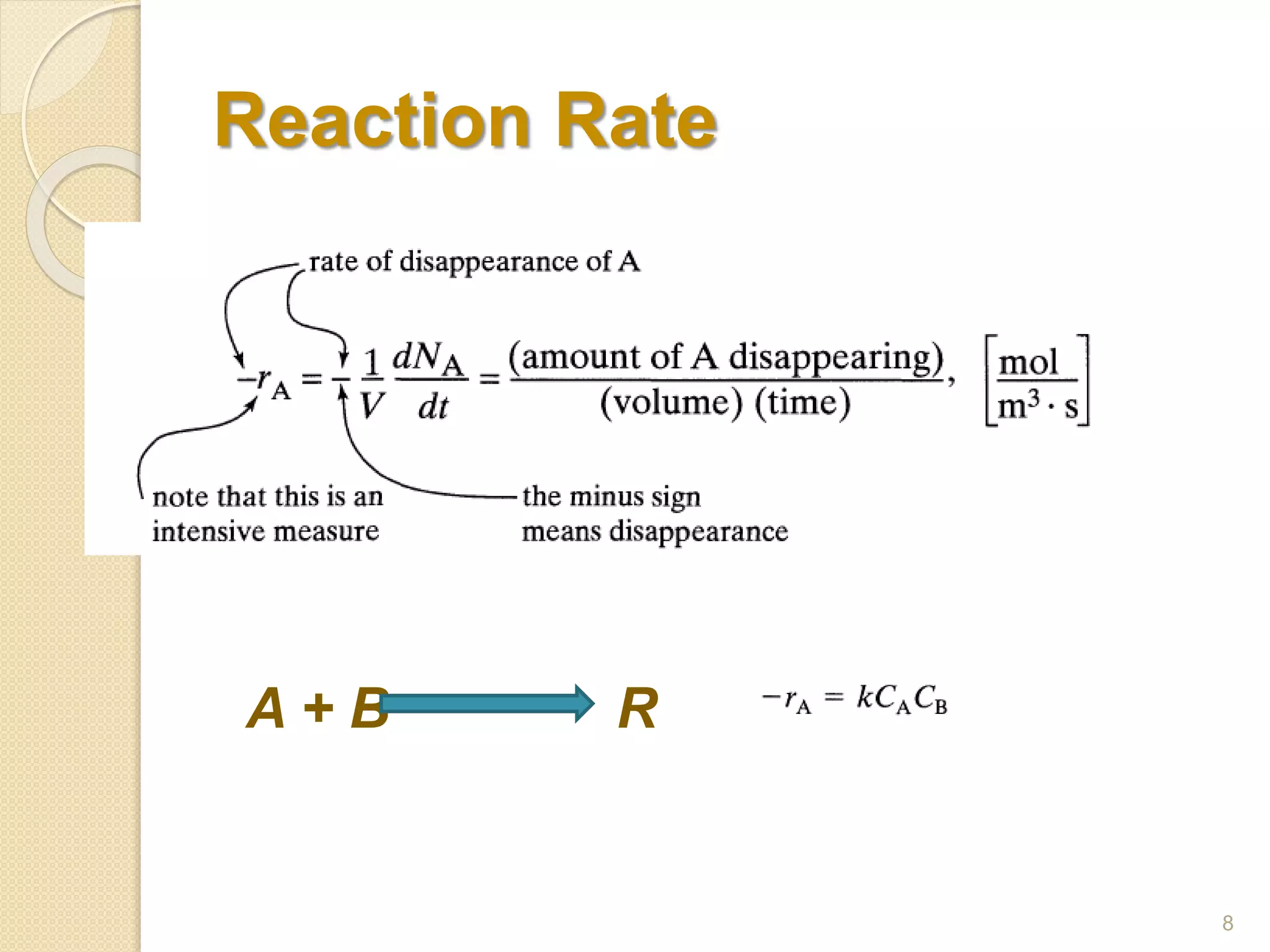
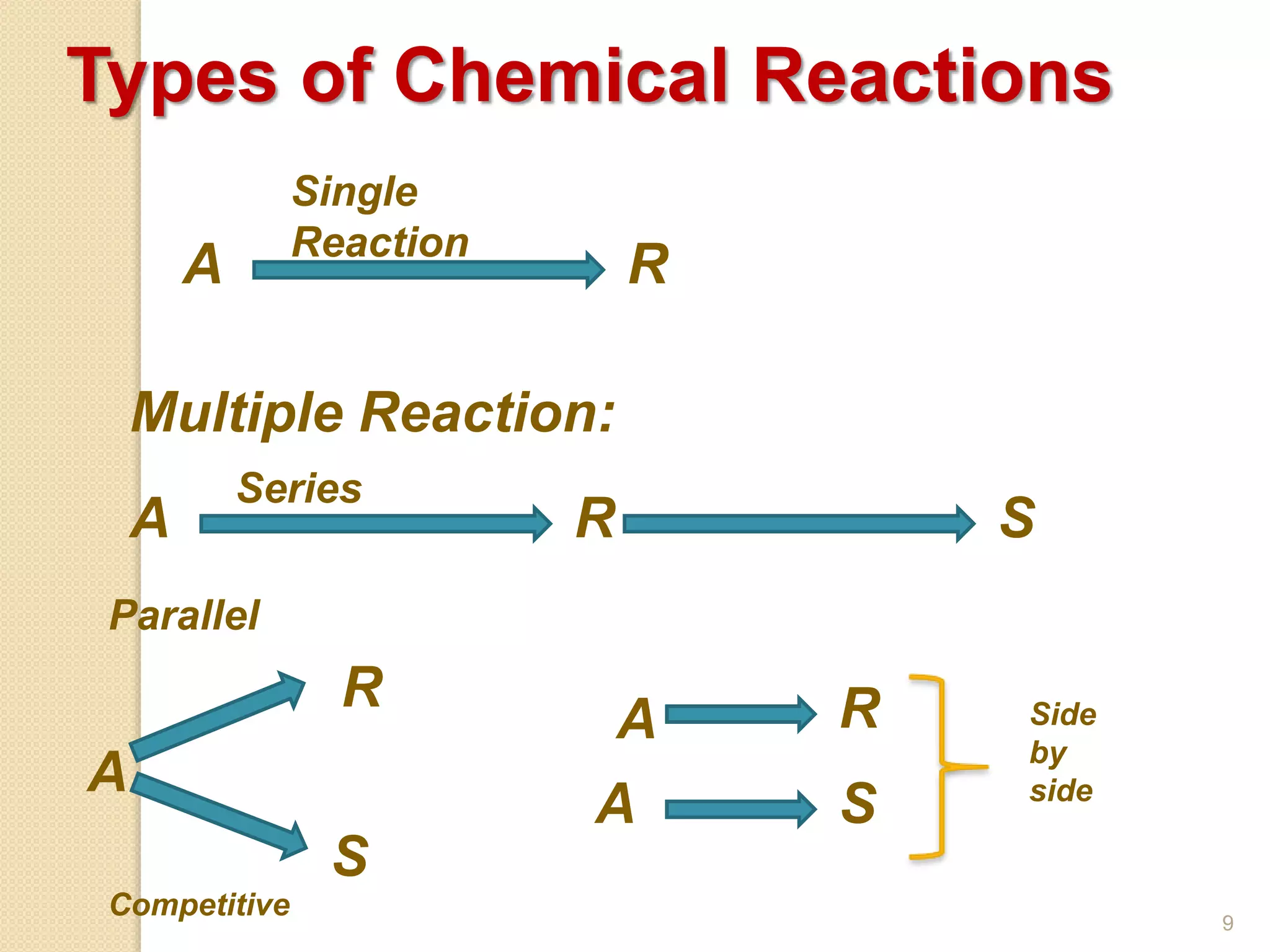
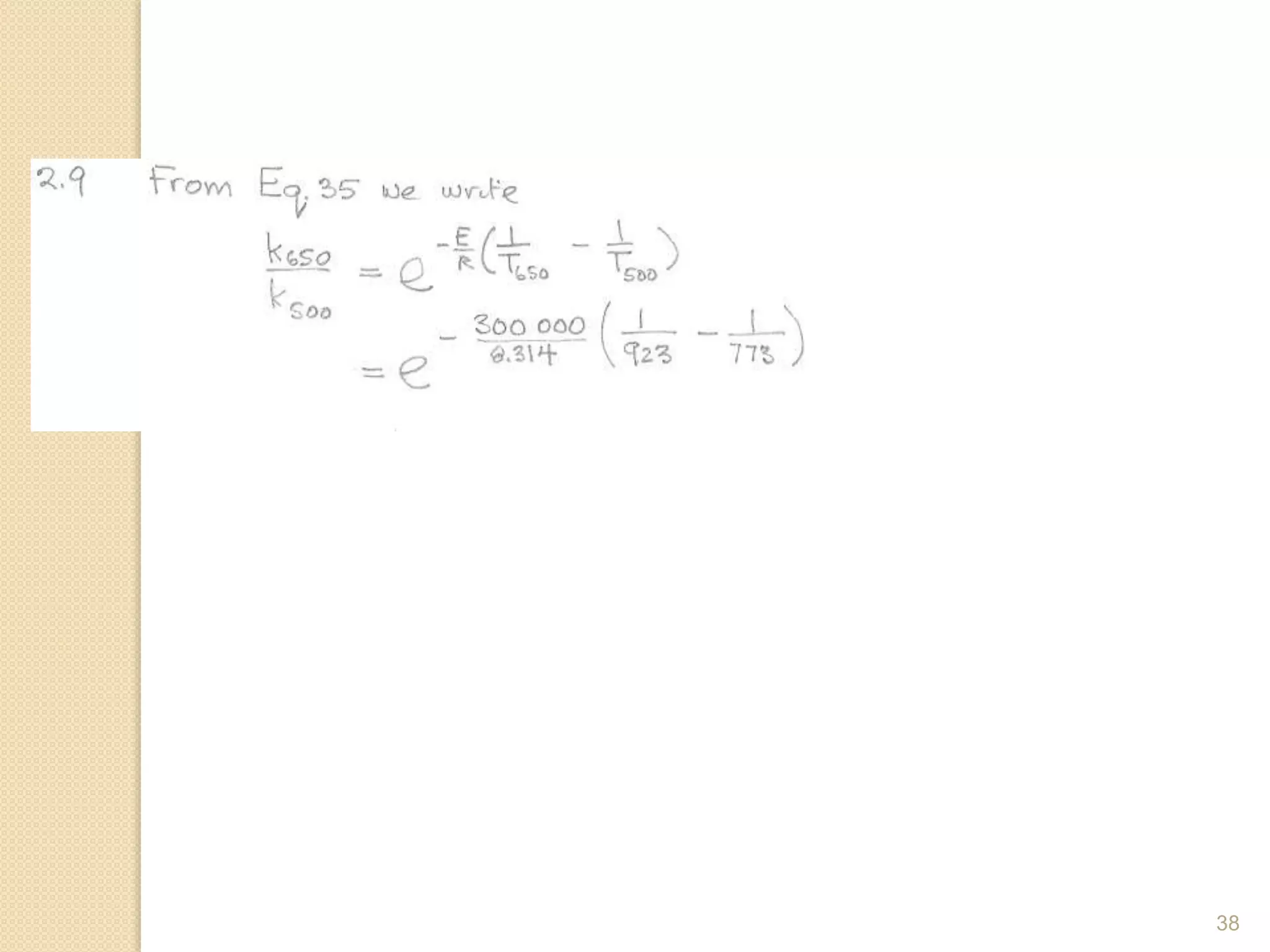Chemical Reaction Engineering (CRE) focuses on the rates and mechanisms of chemical reactions and reactor design. Key topics include mole balance, rate laws, stoichiometry, and reaction orders, which are essential for understanding reaction dynamics. The document also details different reactor types and emphasizes the importance of temperature in reaction rates, particularly using Arrhenius' law.


![Order of Reaction
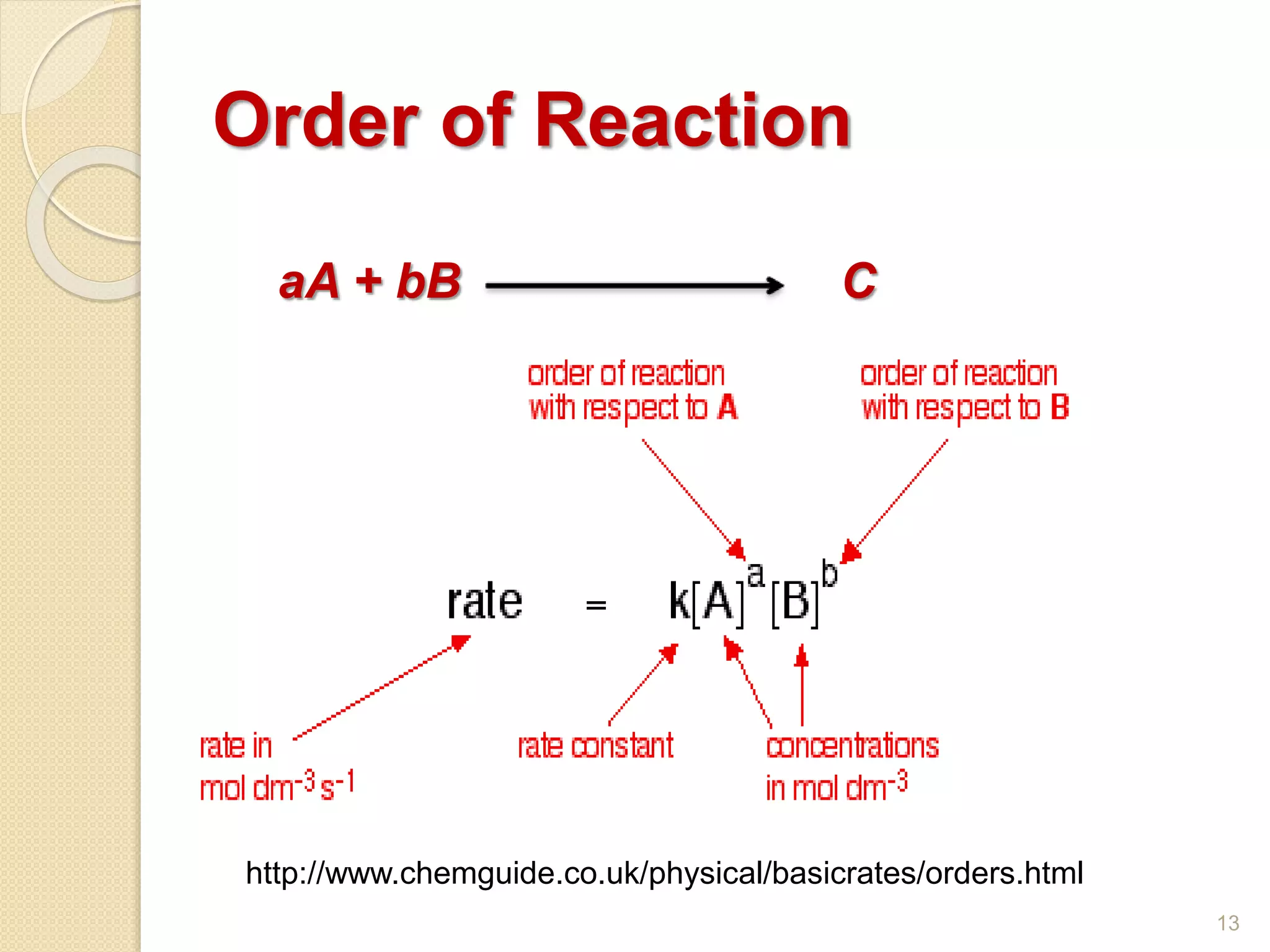
In chemical kinetics, the order of
reaction with respect to a given substance
(such as reactant, catalyst or product) is
defined as the index, or exponent, to which
its concentration term in the rate
equation is raised.
r = [A]x [B]y
[A], [B], are concentrations,
x for substance A & y for substance B, the
reaction orders/ partial reaction orders).
Overall reaction order is
x + y + .... 14](https://image.slidesharecdn.com/lecture3kineticsofhomogeneousreactions-190216170946/75/Lecture-3-kinetics-of-homogeneous-reactions-14-2048.jpg)