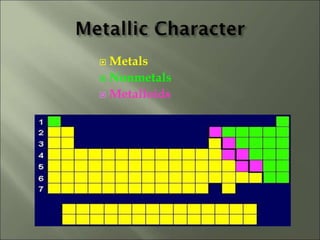
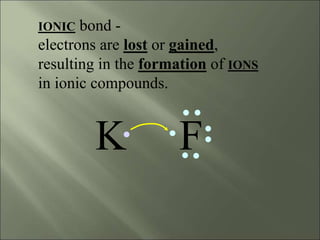
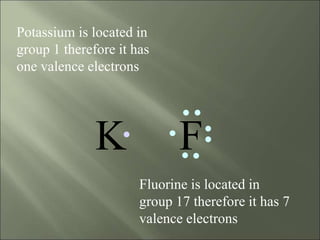
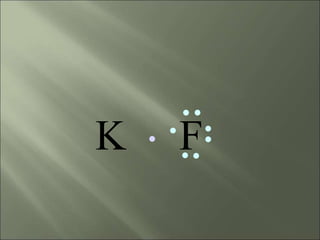
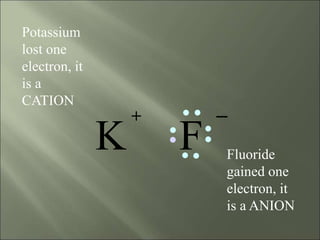
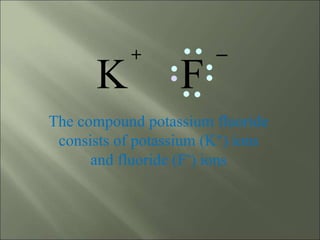
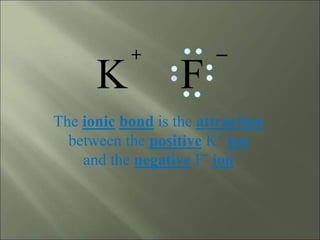
This document discusses ionic bonding, which occurs when atoms gain or lose electrons to achieve stable full outer electron shells of eight electrons. Metals tend to form cations by losing electrons, becoming positively charged ions. Nonmetals tend to form anions by gaining electrons, becoming negatively charged ions. Ionic bonds form when these opposite ions are attracted to each other, such as between sodium and chloride ions to form sodium chloride or table salt.