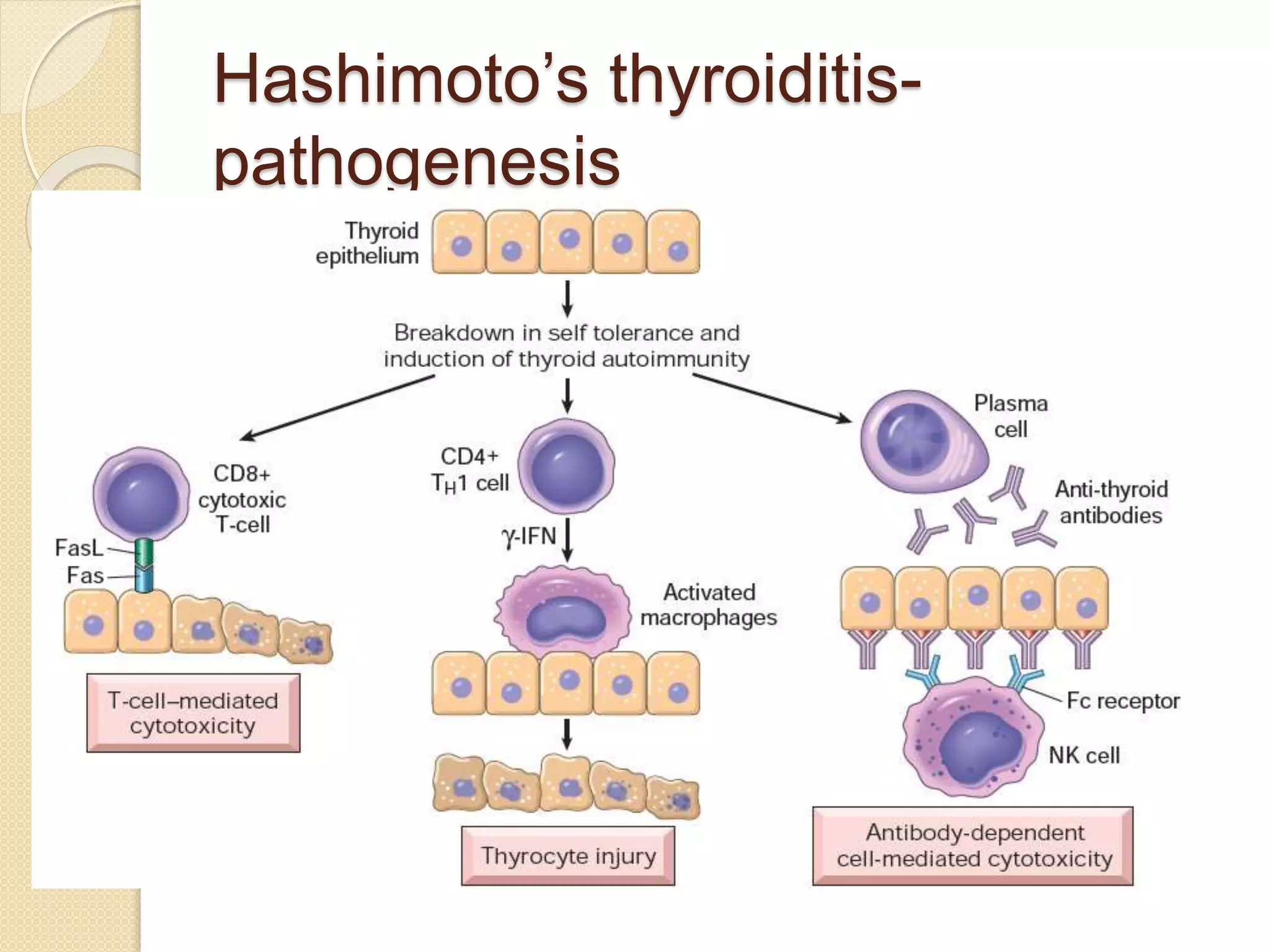
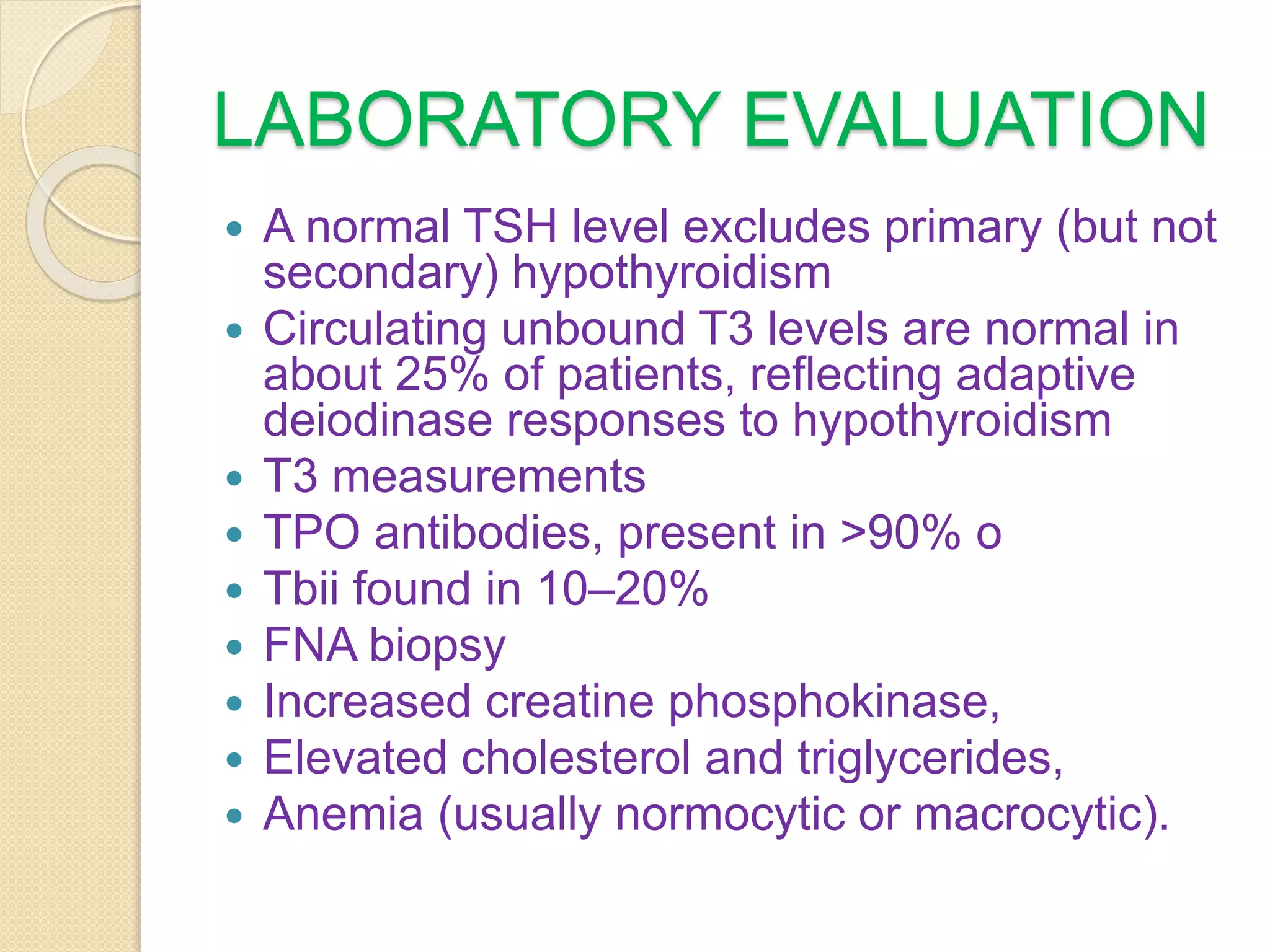
Hypothyroidism is a condition characterized by thyroid hormone deficiency. It ranges from subclinical to myxedema coma. Common causes include iodine deficiency, autoimmune disease like Hashimoto's thyroiditis, and treatment of hyperthyroidism. Symptoms vary but can include fatigue, dry skin, weight gain, constipation, joint pain, and cognitive impairment. Diagnosis is made through blood tests of thyroid hormones and TSH. Treatment involves lifelong levothyroxine replacement therapy to normalize TSH levels. The dosage needs monitoring and adjustment based on repeat testing.