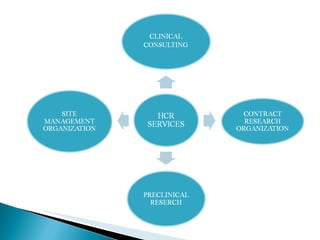
Human Care Research is an independent clinical research organization located in Mumbai, India that provides a wide range of clinical trial services including project management, clinical operations, preclinical operations, data management, medical writing, and regulatory services. It has a network of qualified principal investigators across India and adheres to ICH/GCP guidelines, Indian GCP, and US FDA regulations. Its goal is to support successful clinical trials and drug development through strict ethics and quality standards.