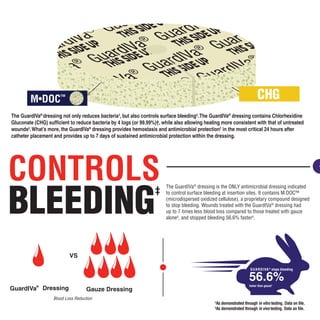
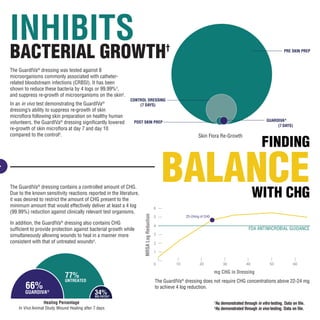
The GuardIVa® dressing reduces 99.99% of bacteria and controls bleeding. It contains chlorhexidine gluconate, which reduces bacteria by 4 logs or 99.99% while allowing wound healing. It also contains microdispersed oxidized cellulose that stops bleeding up to 7 times faster than gauze alone. Additionally, the dressing suppresses bacterial re-growth on skin over 10 days compared to a control.