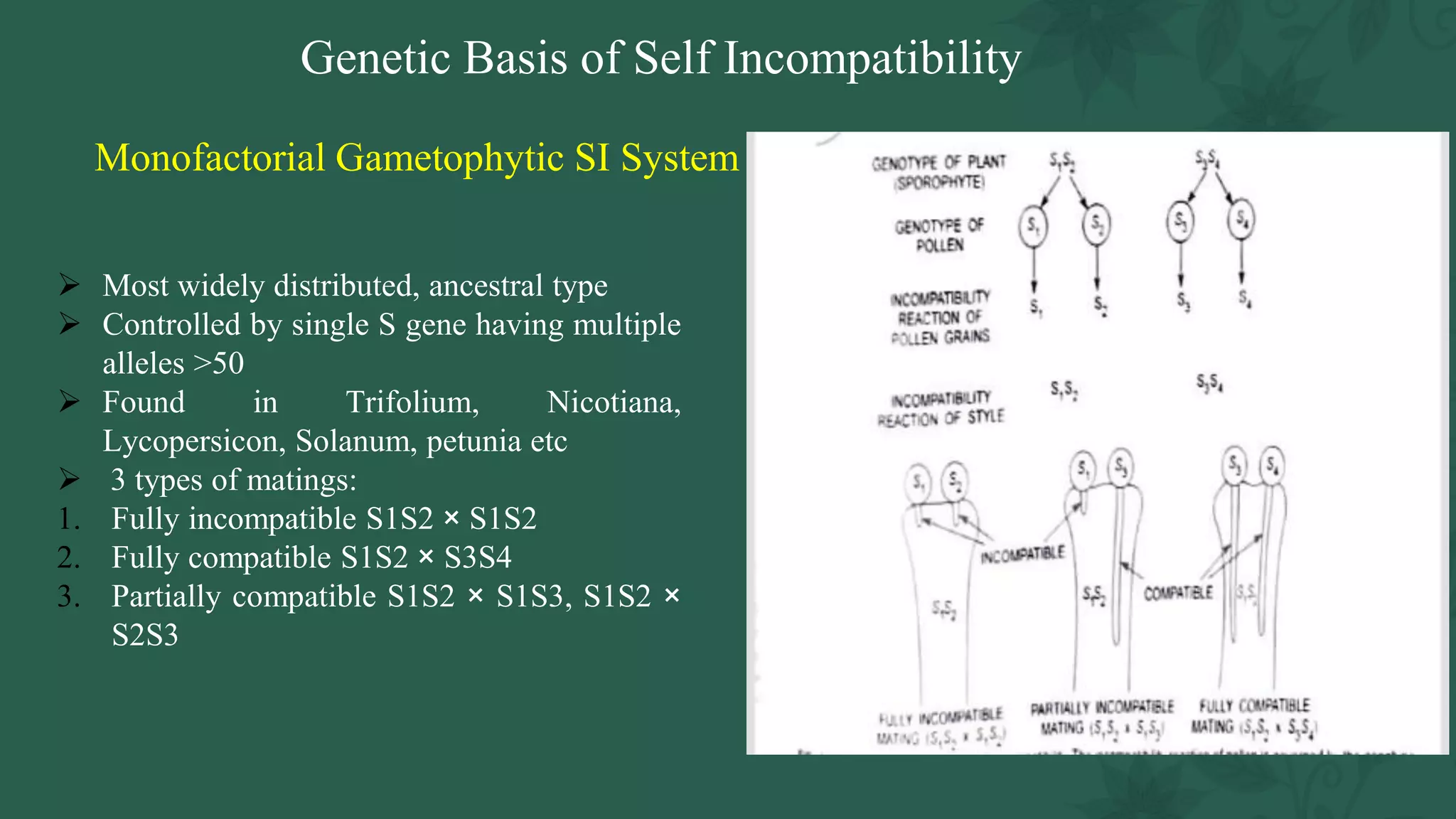
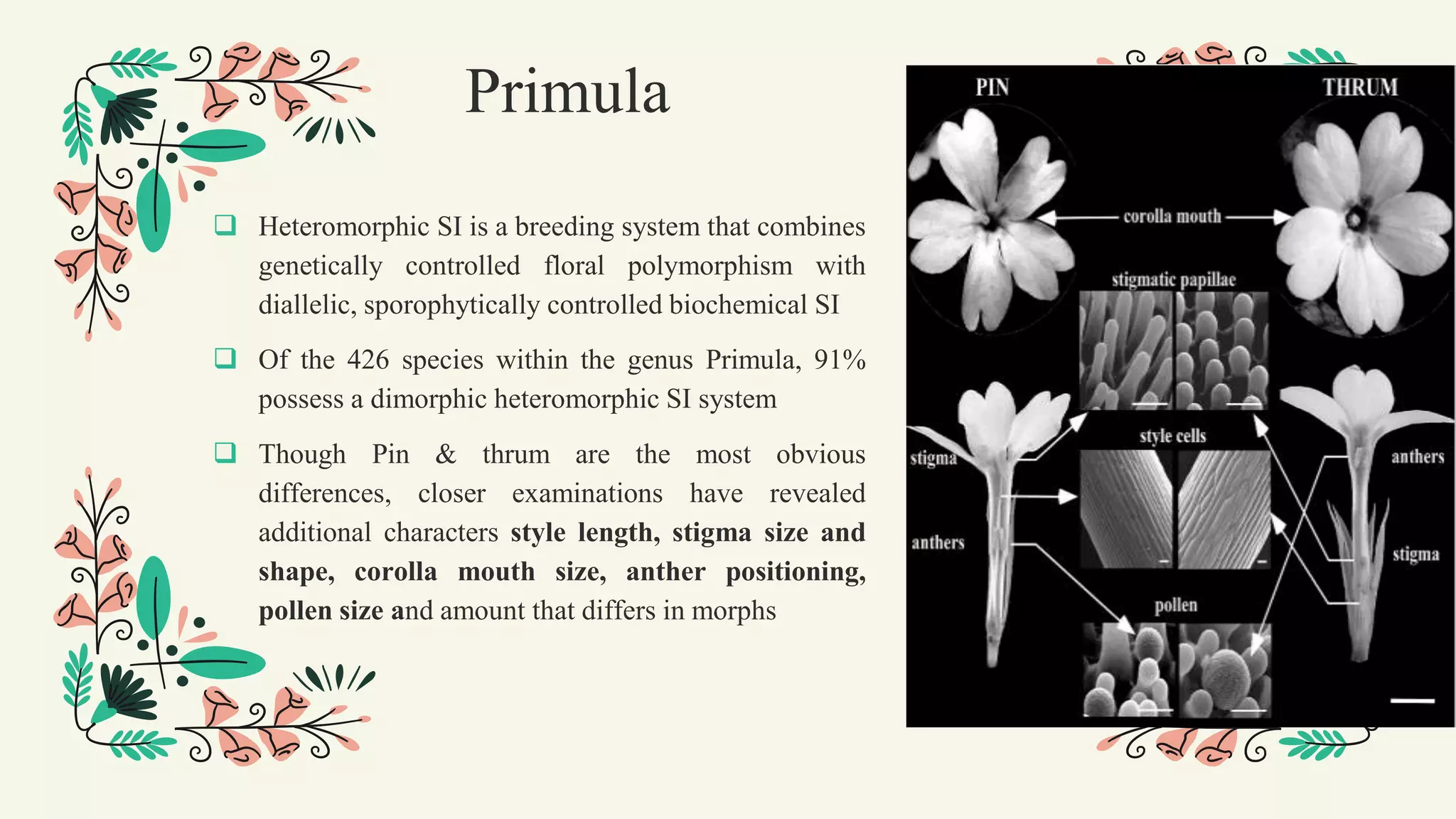
This document discusses genetic, biochemical, and molecular mechanisms of self-incompatibility in plants and factors that can cause its breakdown. It begins by introducing self-incompatibility as the inability of a plant to set seed after self-pollination. It then covers classifications of self-incompatibility, proposed hypotheses for its mechanisms, and genetic bases including mono-and bifactorial gametophytic systems. The document discusses molecular bases in various plant families, including recognition systems in Brassicaceae and Solanaceae. It concludes by outlining factors that can lead to the breakdown of self-incompatibility, such as polyploidy, mutations affecting enzymatic activity, and temporary suppression methods.