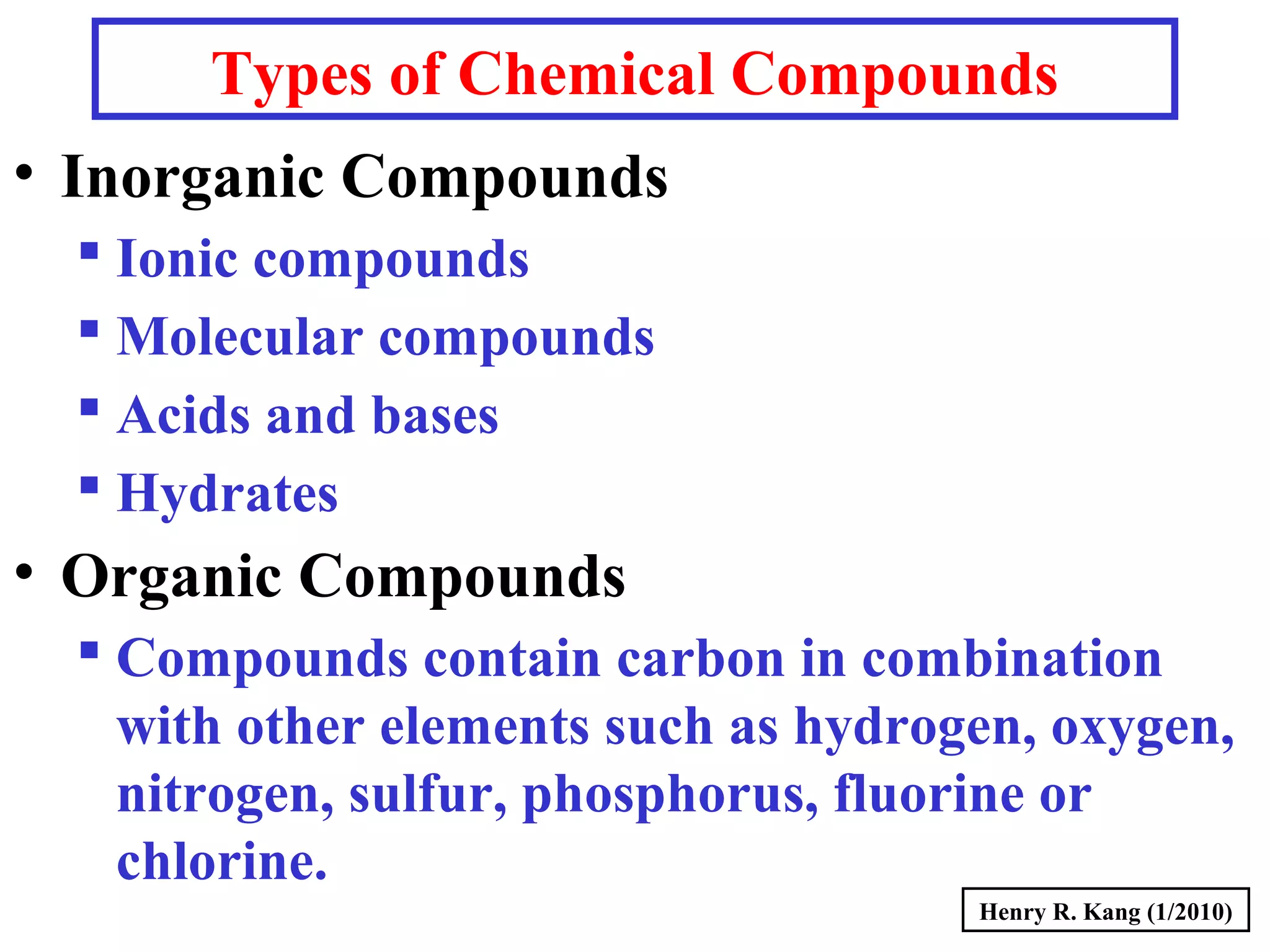
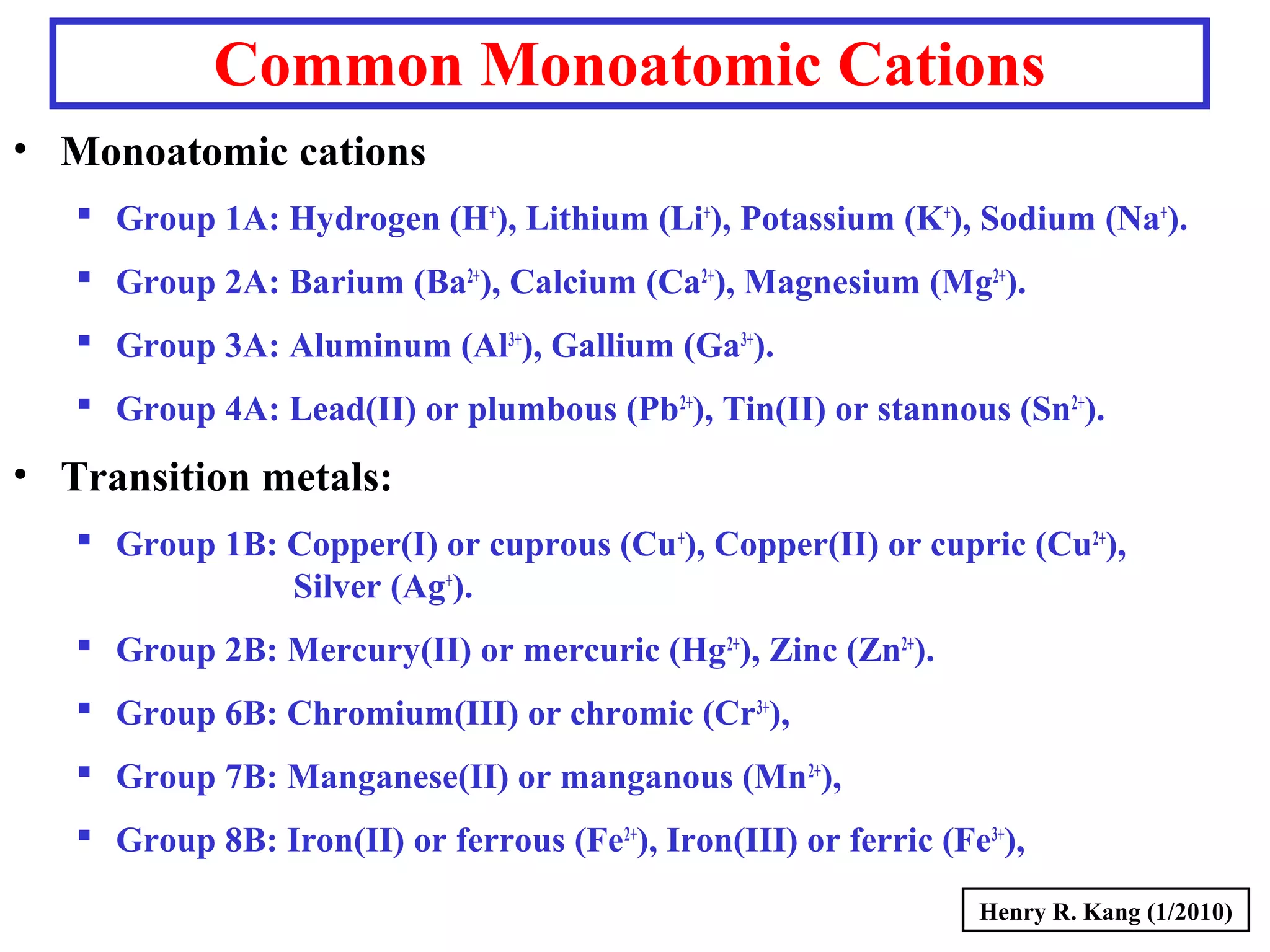
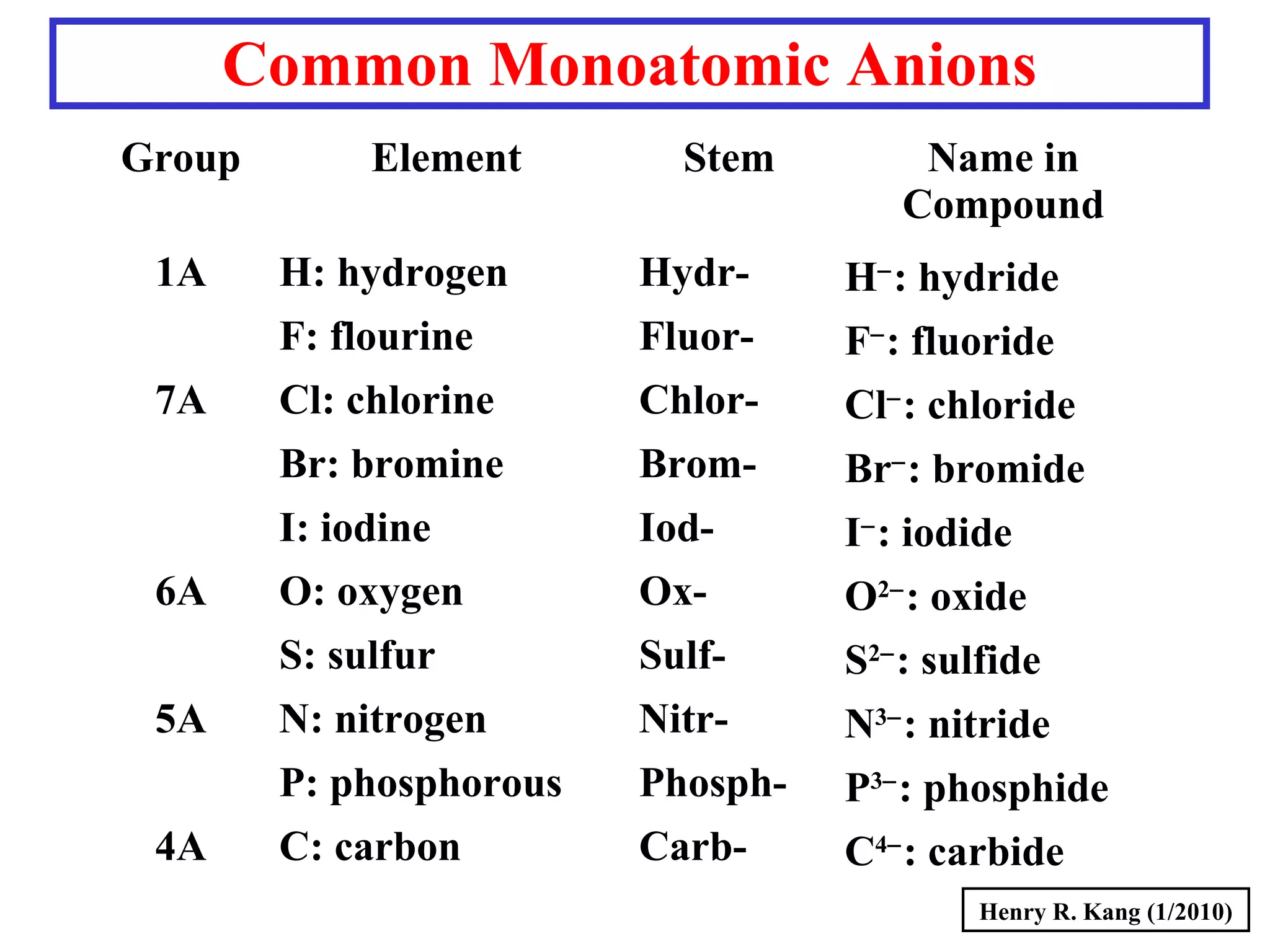
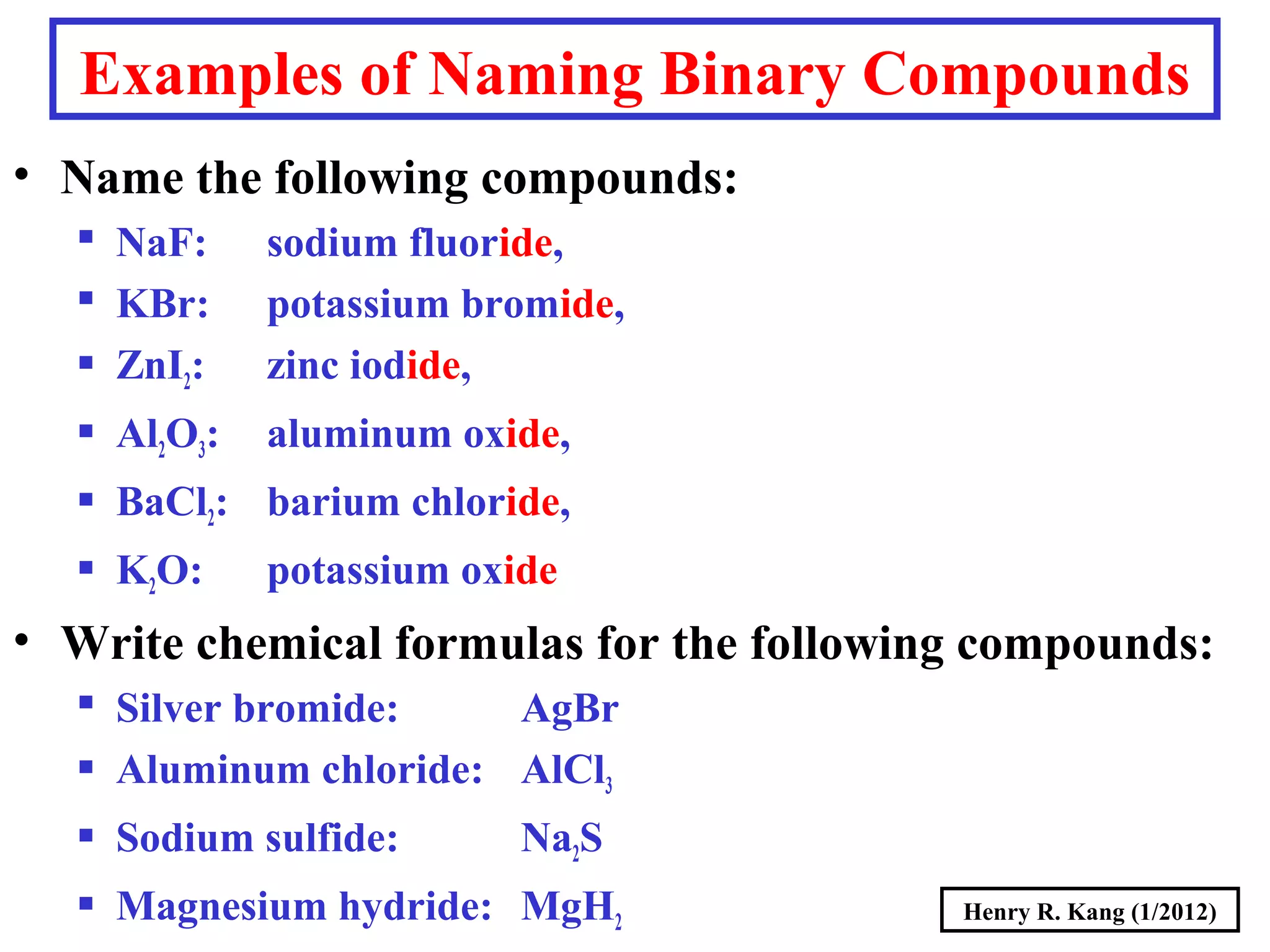
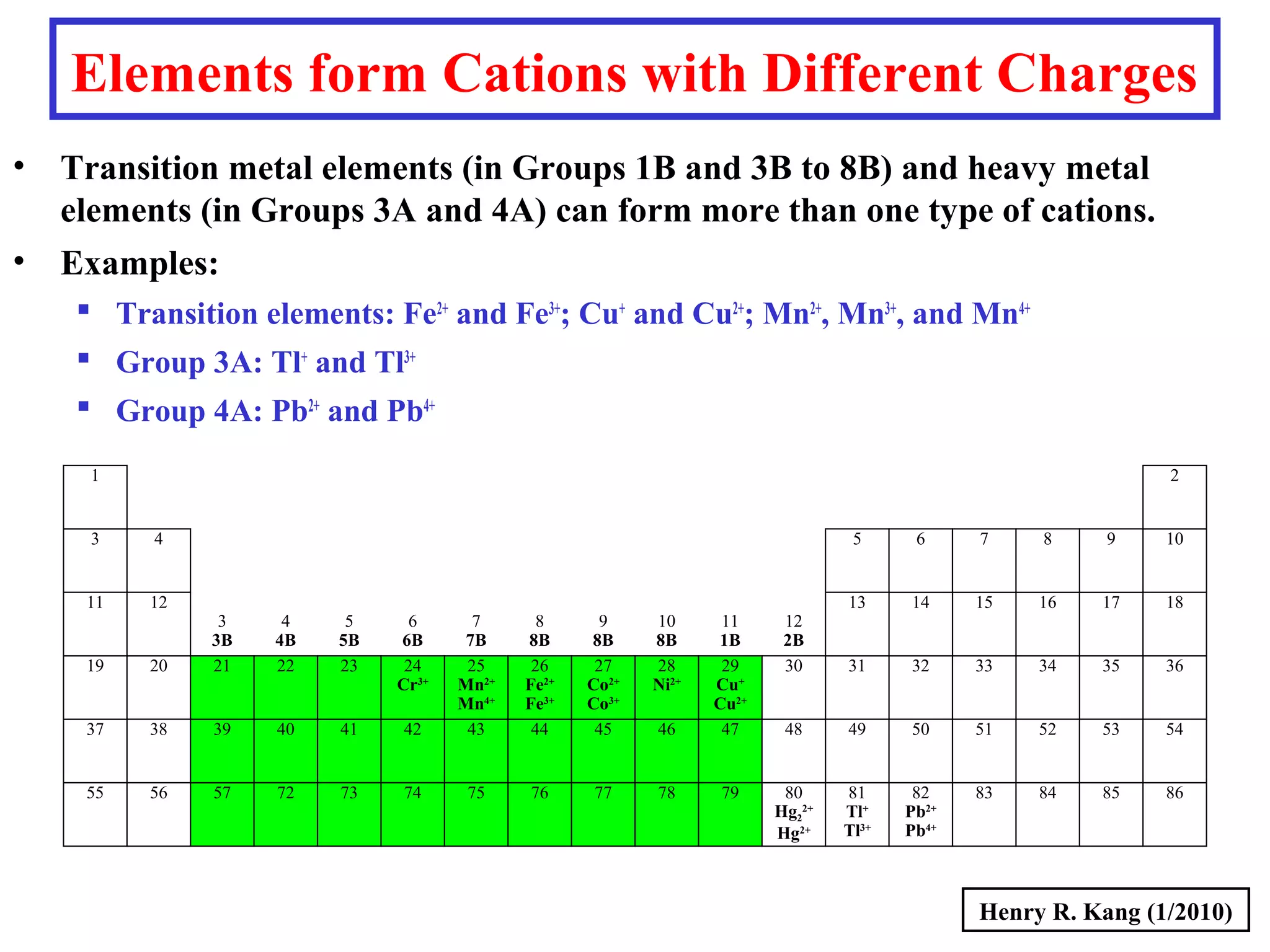
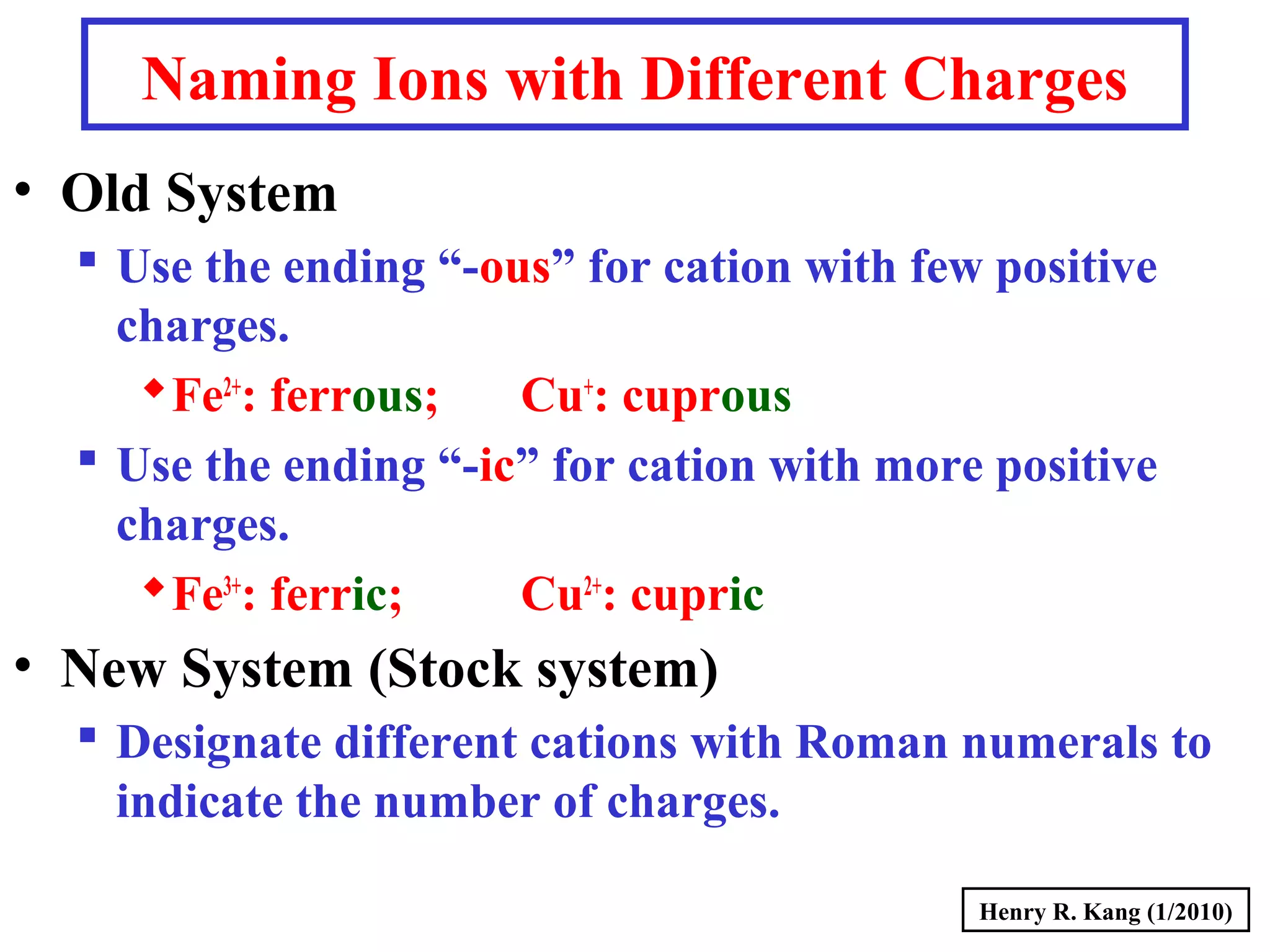
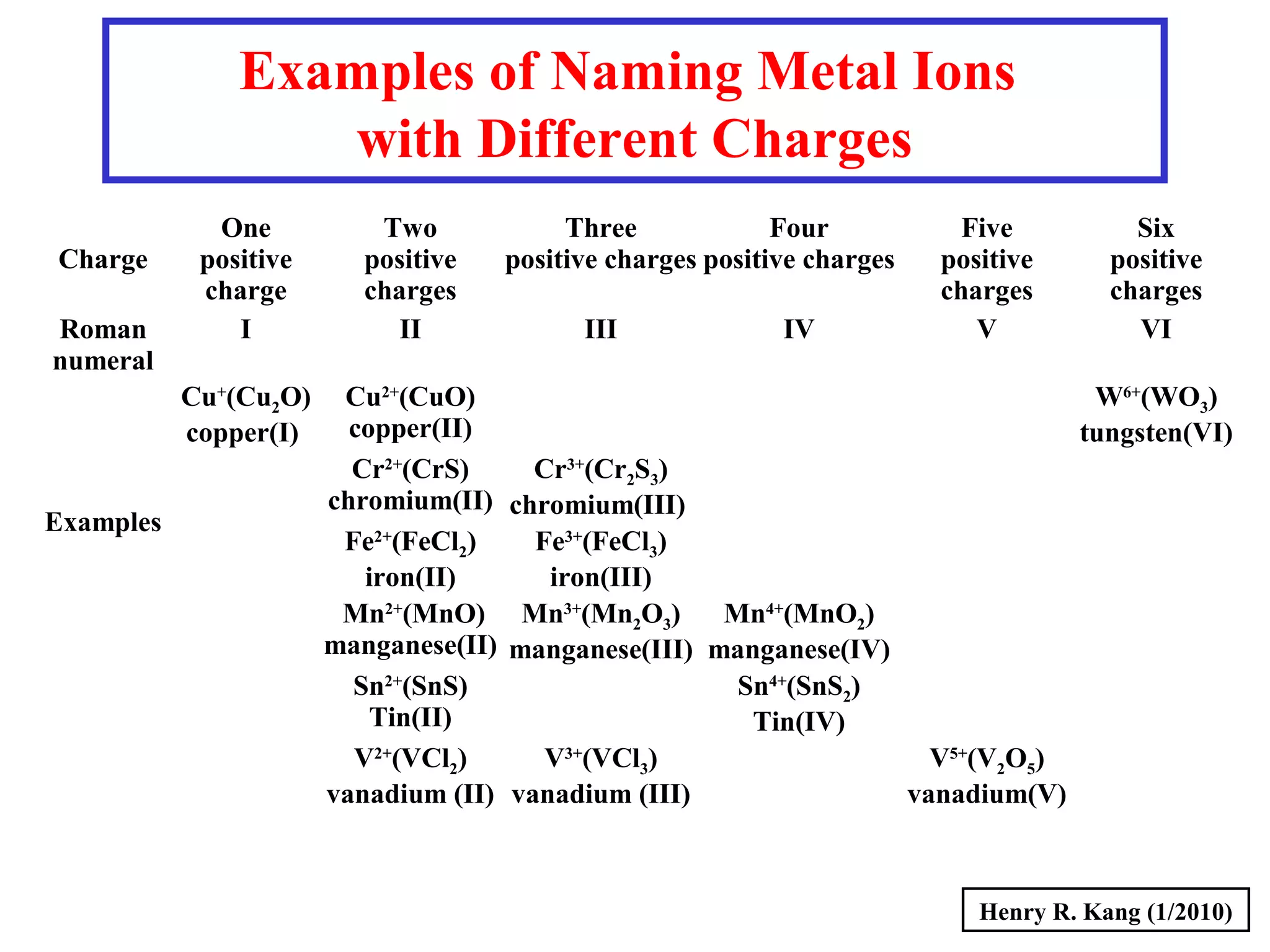
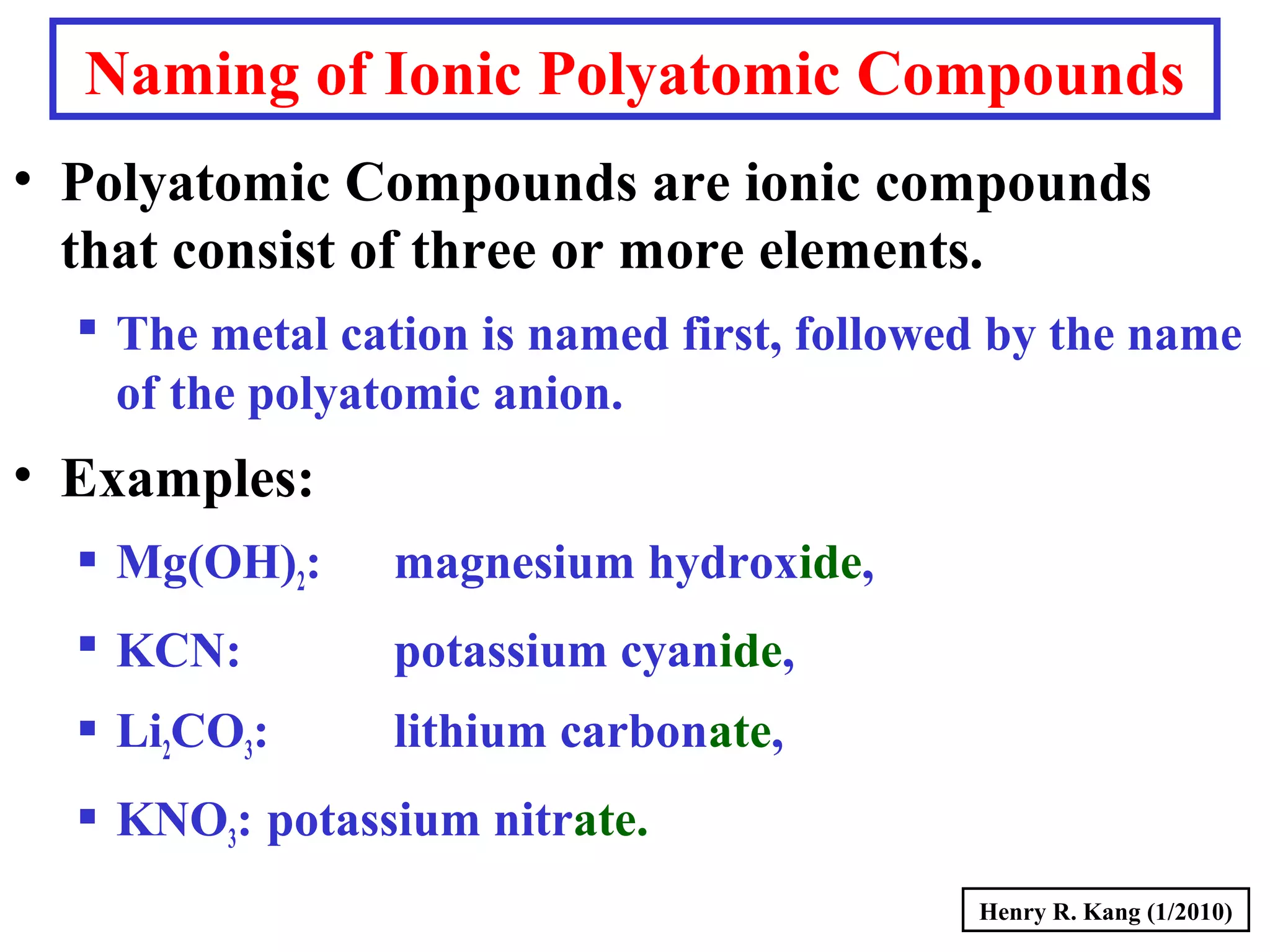
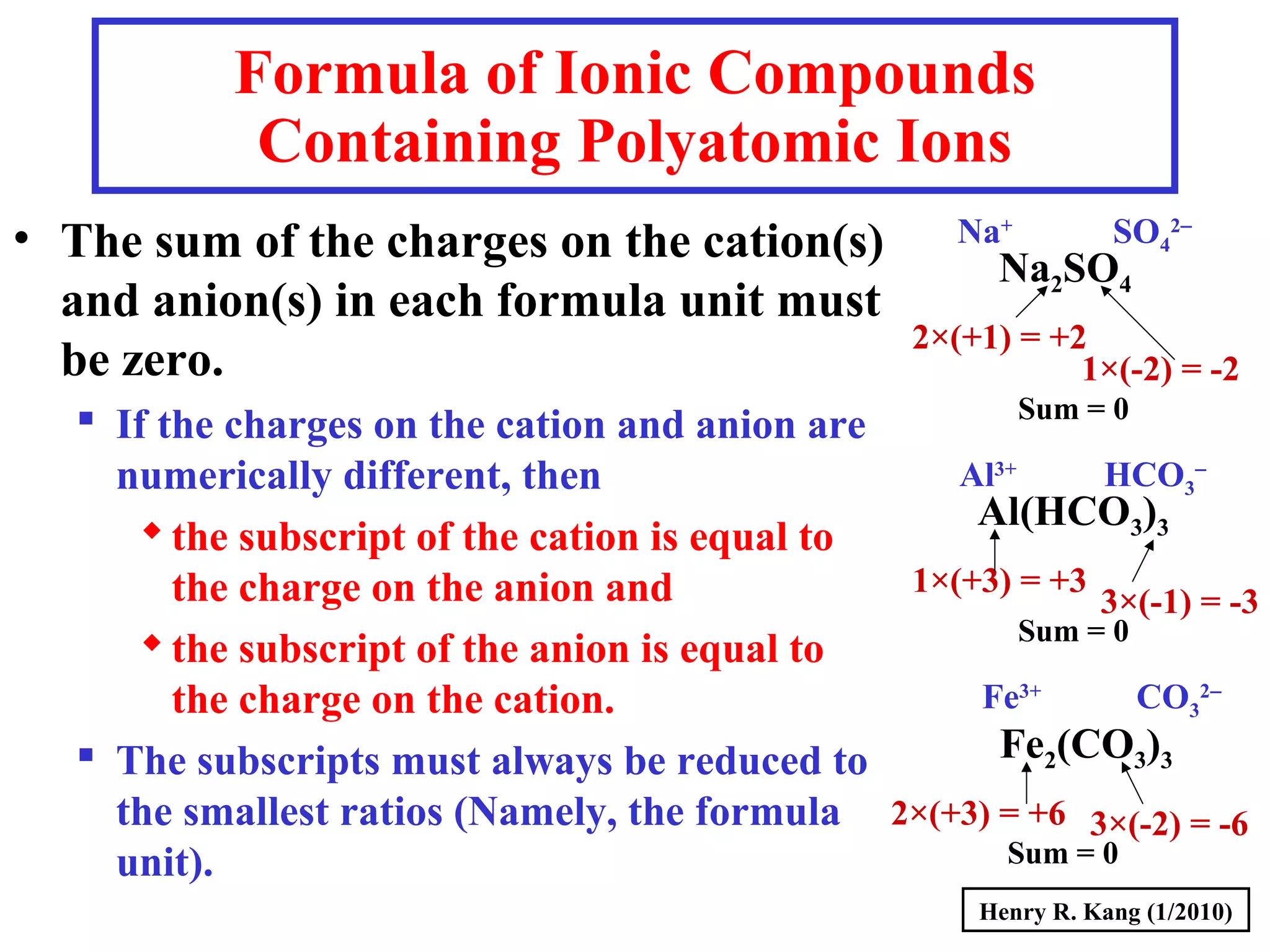
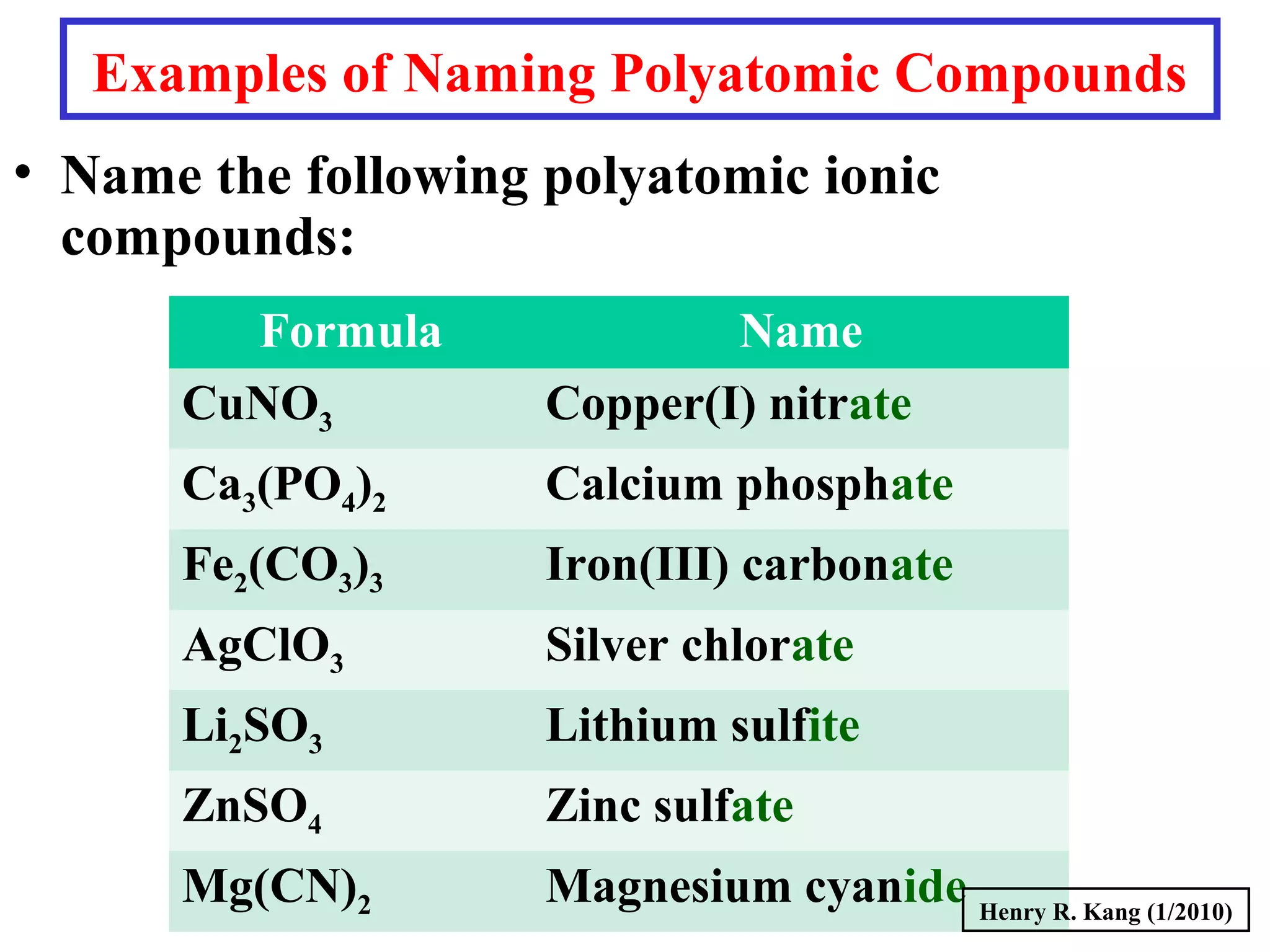
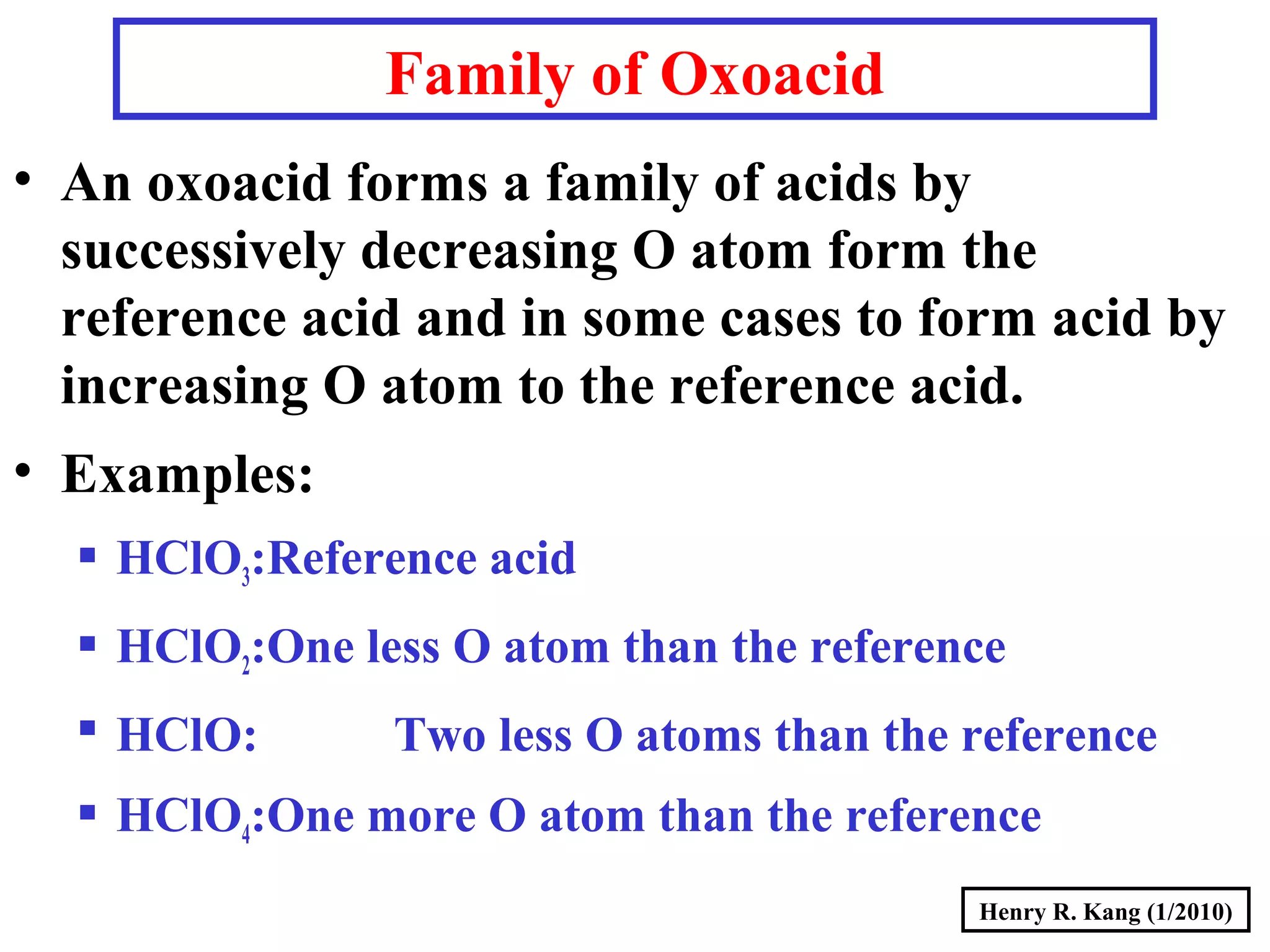
This document provides an overview of chemical nomenclature and naming conventions for different types of chemical compounds. It discusses naming rules for ionic compounds, molecular compounds, acids, and bases. For ionic compounds, it describes how to name binary ionic compounds and polyatomic compounds based on the cation and anion present. It also addresses naming metal ions with different oxidation states. For molecular compounds, it outlines using prefixes to indicate the number of each type of atom. The document concludes with sections on naming simple acids based on replacing the nonmetal element ending with "-ic acid" and an overview of oxoacids and bases.





![Some Common Polyatomic Anions and Cations
Anion (One negative charge) Formula Anion (Two negative charges) Formula
Acetate CH3COO–
Carbonate CO3
2–
Chlorate ClO3
–
Chromate CrO4
2–
Chlorite ClO2
–
Dichromate Cr2O7
2–
Cyanide CN–
Hydrogen phosphate (biphosphate) HPO4
2–
Dihydrogen phosphate H2PO4
–
Oxalate C2O4
2–
Hydrogen carbonate (bicarbonate) HCO3
–
Peroxide O2
2–
Hydrogen oxalate HC2O4
–
Sulfate SO4
2–
Hydrogen sulfate (bisulfate) HSO4
–
Sulfite SO3
2–
Hydrogen sulfite (bisulfite) HSO3
–
Silicate SiO3
2–
Hydrogen sulfide HS–
Anion (Three negative charges)
Hydroxide OH–
Arsenate AsO4
3–
Hypochlorite ClO–
Borate BO3
3–
Nitrate NO3
–
Phosphate PO4
3–
Nitrite NO2
–
Phosphite PO3
3–
Perchlorate ClO4
–
Cation
Permanganate MnO4
–
Ammonium NH4
+
Thiocyanate SCN–
Mercurous [or Mercury(I)] Hg2
2+Henry R. Kang (1/2010)](https://image.slidesharecdn.com/63bd70f9-fb54-4fb2-b359-4254dec54f2e-150322163323-conversion-gate01/75/GC-S010-Nomenclature-15-2048.jpg)









![Naming Oxoacid and Oxoanion: HNO3
Oxoacid Oxoanion
Example Example
Representative
“-ic” acid
“-ous” acid
“hypo-” “-ous” acid
“-ate”
“-ite”
“hypo-” “-ite”
HNO3
nitric acid
HNO2
nitrous acid
HNO
hyponitrous acid
Removal of
all H ions
NO3
−
nitate
NO2
−
nitrite
NO−
hyponitrite
-[O]
-[O]
Reference acid
Henry R. Kang (8/2010)](https://image.slidesharecdn.com/63bd70f9-fb54-4fb2-b359-4254dec54f2e-150322163323-conversion-gate01/75/GC-S010-Nomenclature-28-2048.jpg)
![Naming Oxoacid & Oxoanion: H2SO4
Oxoacid Oxoanion
Example Example
Representative
“-ic” acid
“-ous” acid
“hypo-” “-ous” acid
“-ate”
“-ite”
“hypo-” “-ite”
H2SO4
sulfuric acid
H2SO3
sulfurous acid
H2SO2
hyposulfurous
acid
Removal of
all H ions
SO4
2−
sulfate
SO3
2−
sulfite
SO2
2−
hyposulfite
-[O]
-[O]
Reference acid
Henry R. Kang (1/2012)](https://image.slidesharecdn.com/63bd70f9-fb54-4fb2-b359-4254dec54f2e-150322163323-conversion-gate01/75/GC-S010-Nomenclature-29-2048.jpg)
![Naming Oxoacids & Oxoanions: H3PO4
Oxoacid Oxoanion
Example Example
Representative
“-ic” acid
“-ous” acid
“hypo-” “-ous” acid
“-ate”
“-ite”
“hypo-”
“-ite”
H3PO4
H3PO3
phosphoric acid
H3PO2
phosphorous acid
hypophosphorous
acid
PO4
3−
Removal
of all H ions
PO3
3−
phosphate
PO2
3−
phosphite
hypophosphite
-[O]
-[O]
Reference acid
Henry R. Kang (8/2010)](https://image.slidesharecdn.com/63bd70f9-fb54-4fb2-b359-4254dec54f2e-150322163323-conversion-gate01/75/GC-S010-Nomenclature-30-2048.jpg)
![Henry R. Kang (1/2010)
Naming Oxoacids & Oxoanion: HBrO3
Example Example
“per-” “-ic” acid
Representative
“-ic” acid
“-ous” acid
“hypo-” “-ous” acid
“per-” “-ate”
“-ate”
“-ite”
“hypo-”
“-ite”
HBrO4
perbromic acid
HBrO3
bromic acid
HBrO2
bromous acid
HBrO
hypobromou
s acid
BrO4
−
perbromate
BrO3
−
bromate
BrO2
−
bromite
BrO−
hypobromite
+[O]
-[O]
-[O]
Reference acid](https://image.slidesharecdn.com/63bd70f9-fb54-4fb2-b359-4254dec54f2e-150322163323-conversion-gate01/75/GC-S010-Nomenclature-31-2048.jpg)
![Henry R. Kang (1/2010)
Examples of Naming Oxoacids & Salts
• Name the following oxoacid, oxoanion, and salts:
H3PO3: Phosphorous acid
One fewer O than the reference phosphoric acid (H3PO4)
IO4
-
: Periodate
One more O than the reference iodic acid (HIO3).
HBrO: Hypobromous acid
Two fewer O atoms than the reference bromic acid (HBrO3).
HSO4
-
: Hydrogen sulfate ion
Cu(NO3)2: Copper(II) nitrate [Stock system]
KH2PO4: Potassium dihydrogen phosphate
NH4ClO3: Ammonium chlorate
Li2SO3: Lithium sulfite (one less O than the reference)](https://image.slidesharecdn.com/63bd70f9-fb54-4fb2-b359-4254dec54f2e-150322163323-conversion-gate01/75/GC-S010-Nomenclature-32-2048.jpg)






