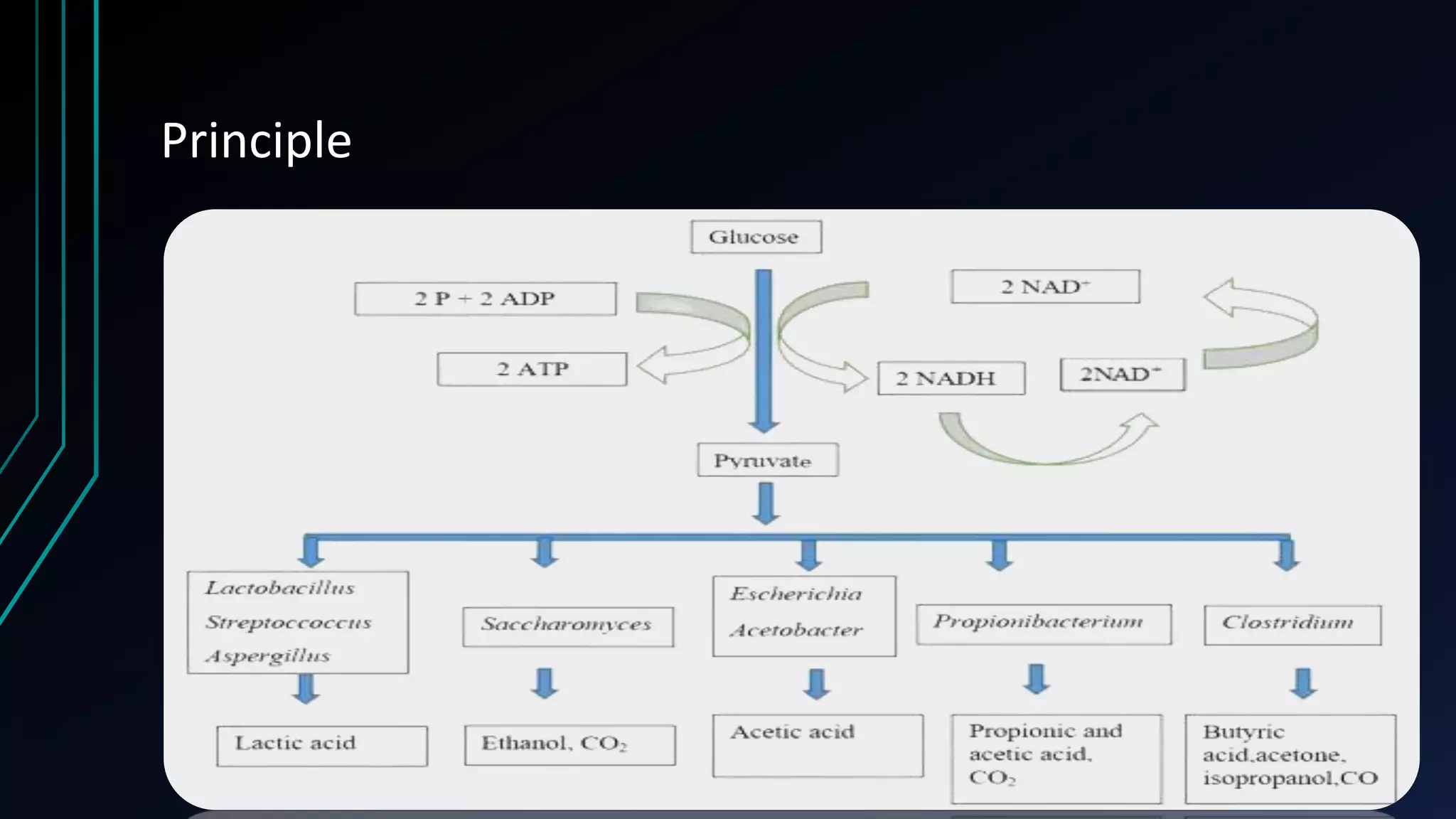
Fermentation is a process where microorganisms convert sugars and starches into other substances in the absence of oxygen. There are several types of fermentation including lactic acid, propionic acid, alcoholic, and butyric acid fermentation. Fermentation has many applications in food production like cheese, wine, and bread. It is also used in medicine to produce antibiotics and vaccines. While fermentation has benefits, it also has limitations like low scale production requiring high costs, risk of contamination, natural variations over time, and production of undesirable end products.