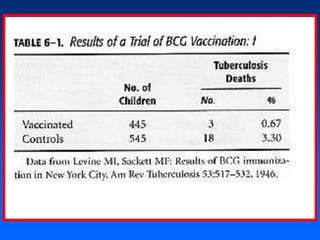
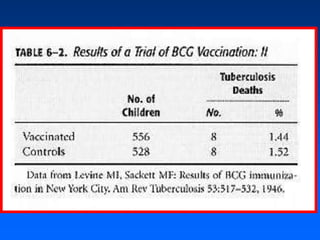
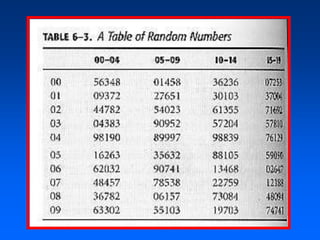
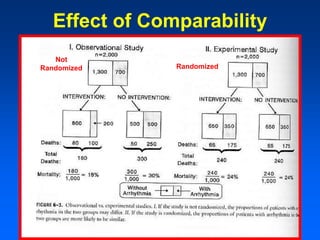
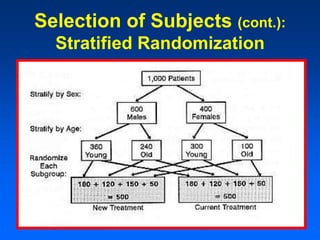
This document discusses experimental study designs, specifically randomized clinical trials. It describes key aspects of randomized trials including multiple experimental groups, blinding techniques, objectives related to public health and clinical practice, and historical examples. Randomized trials are identified as the ideal design for evaluating new interventions by comparing outcomes between randomized treatment groups to eliminate selection bias. Key aspects covered include selection and stratification of subjects, data collection on variables like treatment received, outcomes, and prognostic profiles, as well as blinding techniques.