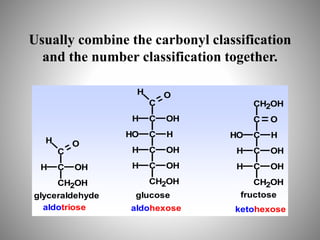
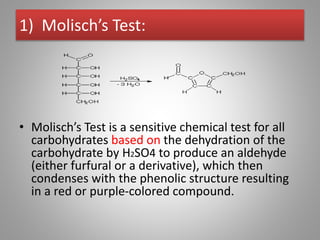
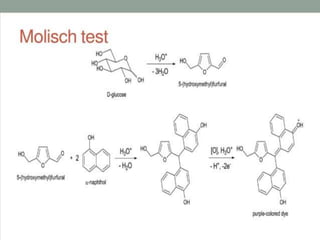
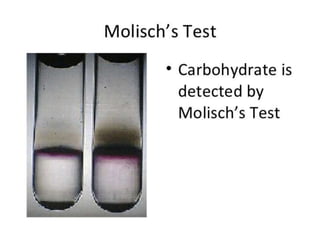
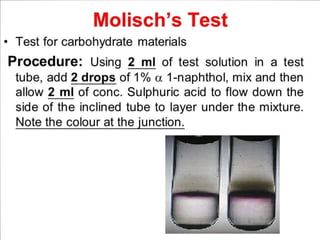
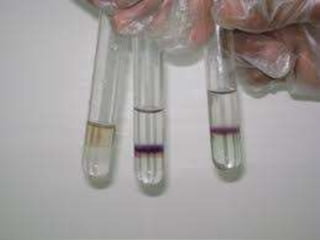
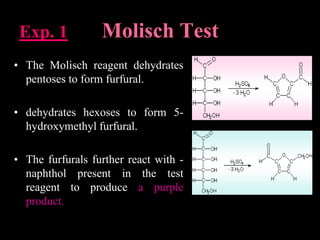
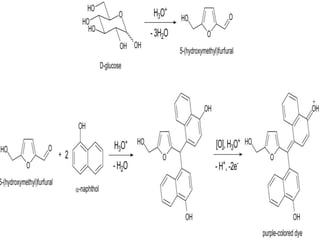
Carbohydrates are organic compounds made of carbon, hydrogen, and oxygen that serve important roles in living systems. They are divided into monosaccharides, disaccharides, and polysaccharides based on their structure. The Molisch test is a general and sensitive test used to detect the presence of all carbohydrates using concentrated sulfuric acid and alpha-naphthol to produce a purple color.