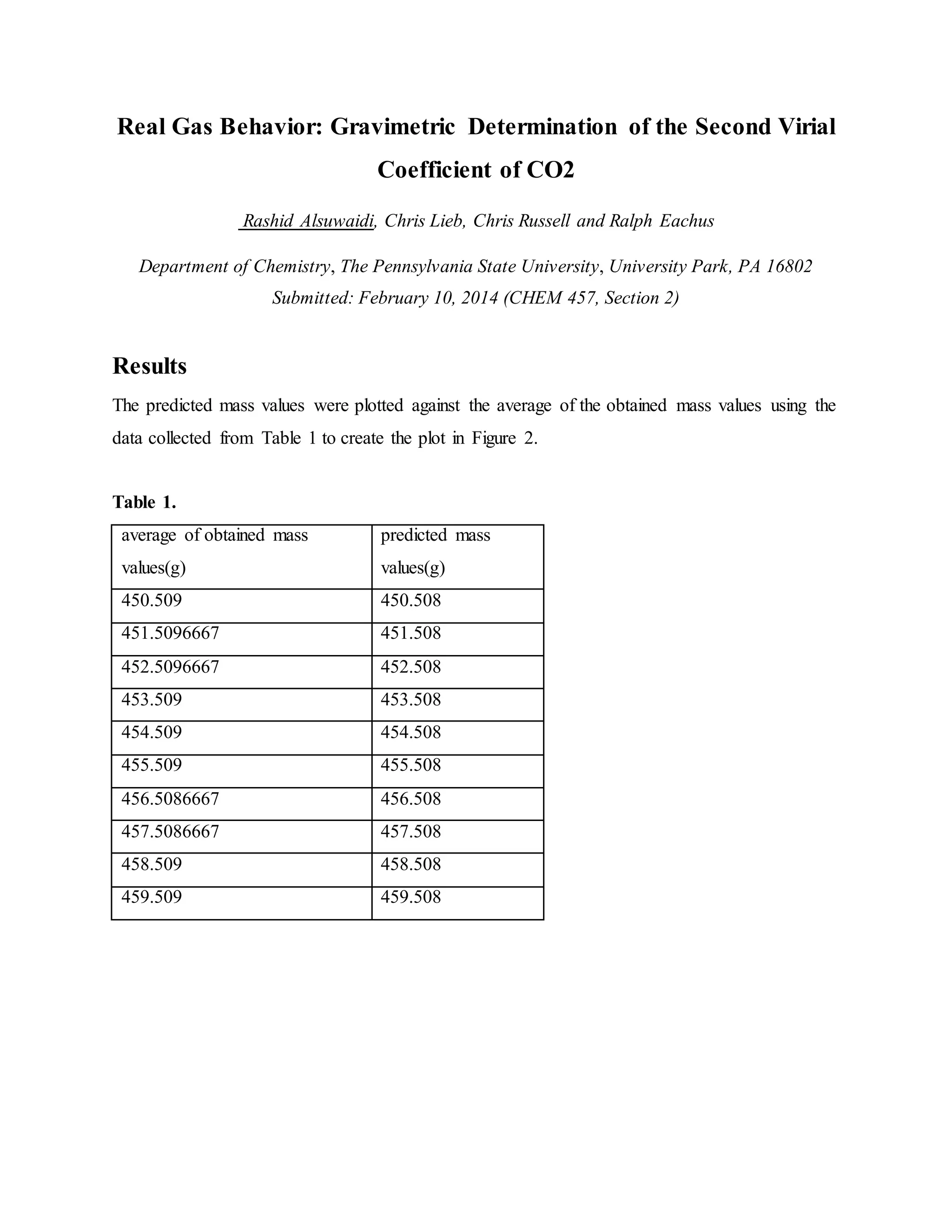
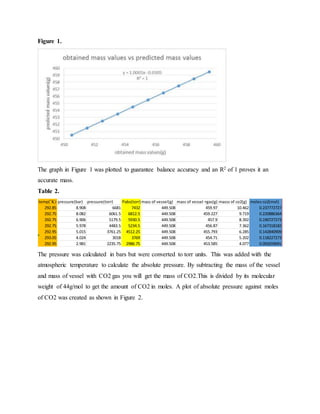
1. The document reports on an experiment to determine the second virial coefficient of carbon dioxide gas through gravimetric measurements at varying pressures and temperatures.
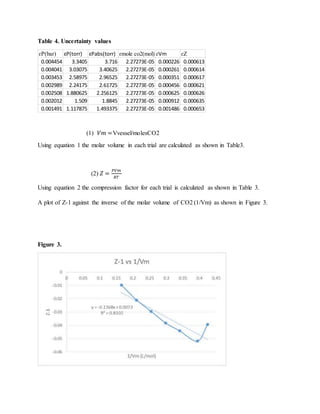
2. A plot of compression factor versus inverse molar volume yielded a second virial coefficient of -0.1368±0.02962 L/mol with an R2 value of 0.8102, indicating some experimental errors.
3. The obtained value has a 22.83% difference from the literature value of -0.1270 L/mol, which is within the experimental uncertainty likely due to temperature fluctuations during the experiment.