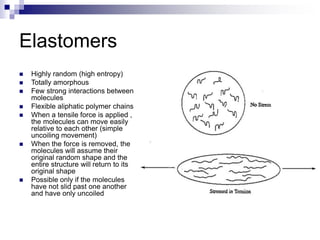
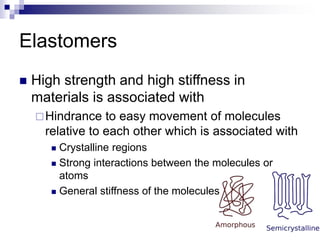
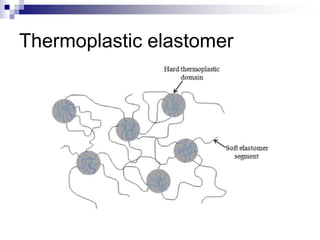
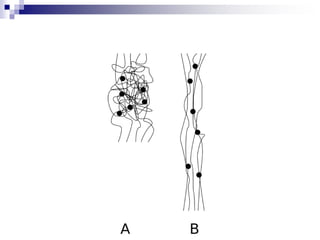
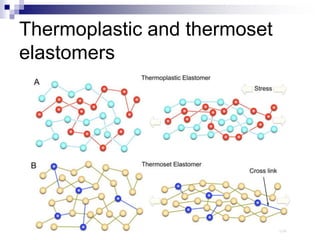
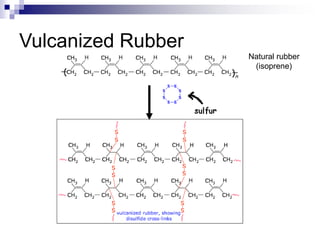
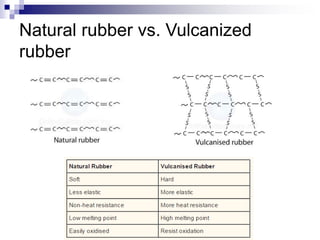
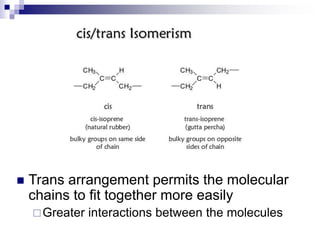
Elastomers are polymers that have over 200% elastic elongation. They can be thermoplastic or thermoset. Elastomers have highly random, flexible chains that allow easy movement under tension, returning to their original shape when tension is removed. High strength comes from hindered movement or strong interactions between molecules. Natural rubber is a cis-polyisoprene elastomer. Vulcanization adds crosslinks for improved properties. Elastomers are used in products requiring cushioning, resilience, or flexibility like gloves, tires, and mattresses.