The document discusses three examples involving Fick's laws of diffusion:
1) Calculating the concentration gradient in a metal diffusion couple.
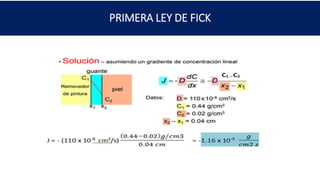
2) Calculating the carbon flux through an iron plate exposed to different carbon concentrations.
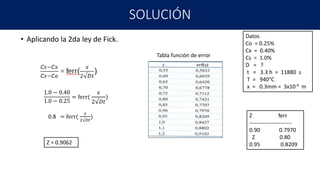
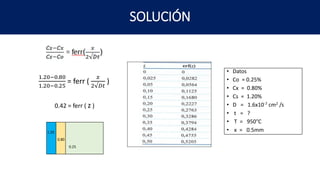
3) Calculating the time needed for cementation to increase the carbon concentration at a given depth in steel exposed to a carbon-rich atmosphere. All three examples show calculations using Fick's first law of diffusion to relate concentration changes to diffusion coefficients and time/distance.