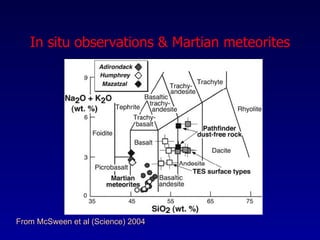
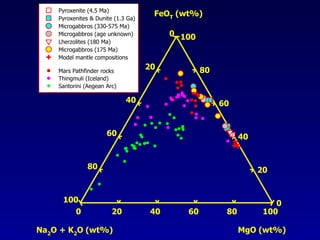
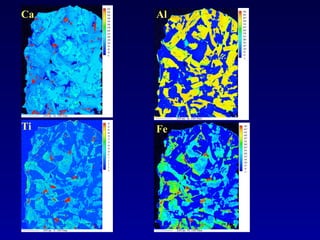
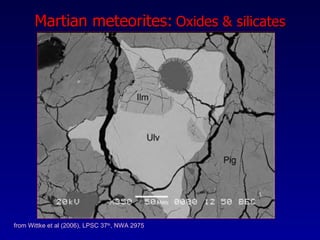
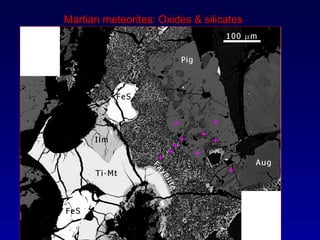
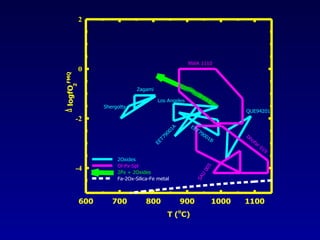
The document discusses redox equilibria and oxygen fugacity in Martian basaltic meteorites. It notes that over 30 pieces of Martian meteorites have been discovered, ranging from 4.3 billion to 150-180 million years old. These meteorites provide insights into Martian geology and chemistry. The document analyzes how phase equilibria and reactions between oxides and silicates can lead to variations in oxygen fugacity during cooling of Martian basalts. It concludes that oxygen fugacity in Martian meteorites is internally buffered by these phase equilibria, rather than resulting from mixing of reduced and oxidized components.