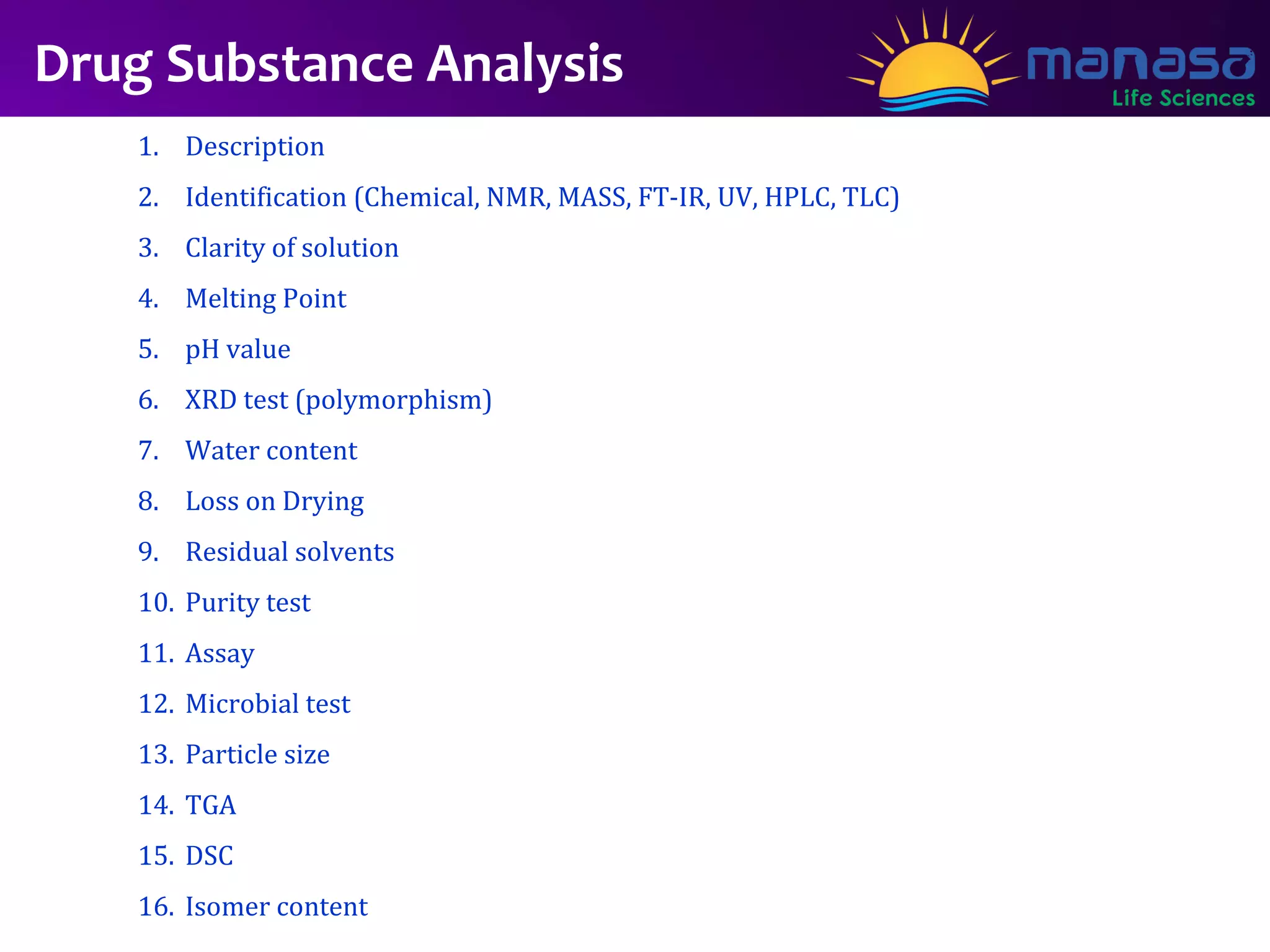
The document provides a detailed overview of the drug canagliflozin, including its uses for treating type 2 diabetes, its approval status, and manufacturing processes. It outlines the synthesis of the drug substance, the analysis required for both drug substance and drug product, and the various excipients used in formulation. Additionally, it includes a case study on drug product development and analysis techniques.