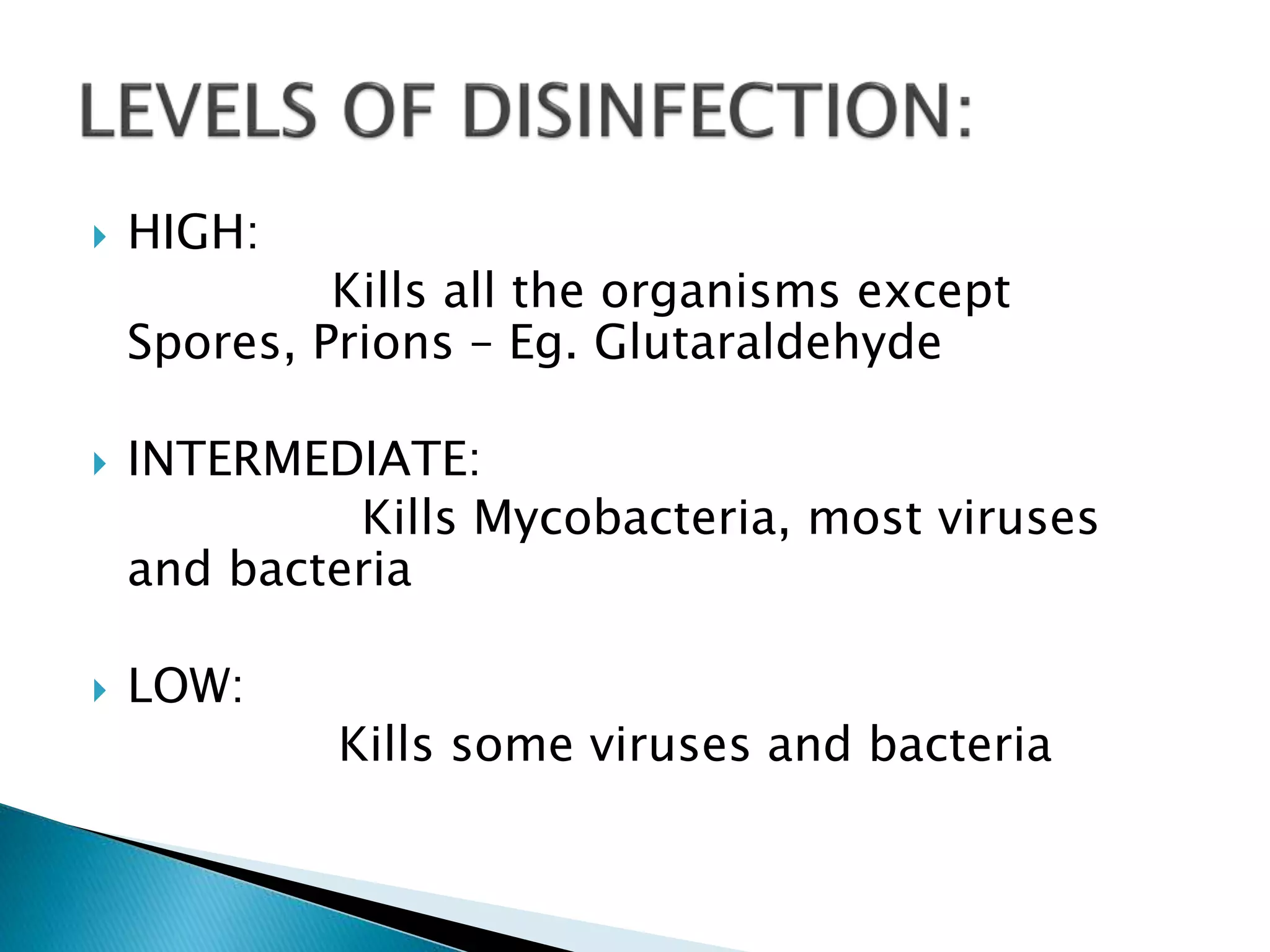
The document provides guidelines for cleaning and disinfection procedures in an operating theatre. It discusses general cleaning including daily scrubbing and disinfecting of surfaces. It also describes procedures for disinfecting instruments using chlorine solutions or glutaraldehyde. Various sterilization methods are outlined like autoclaving of linen and steam sterilization of instruments. The document also discusses sampling and culturing techniques to test the cleanliness of surfaces in the operating theatre.