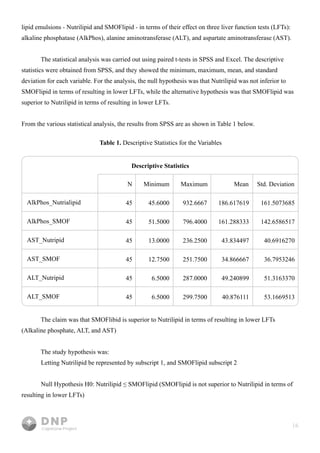
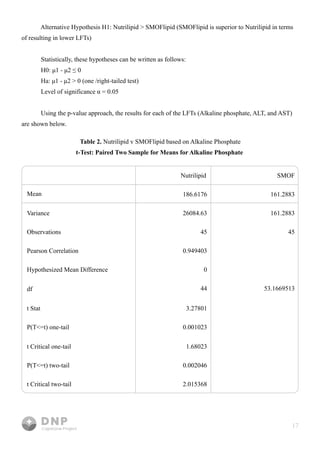
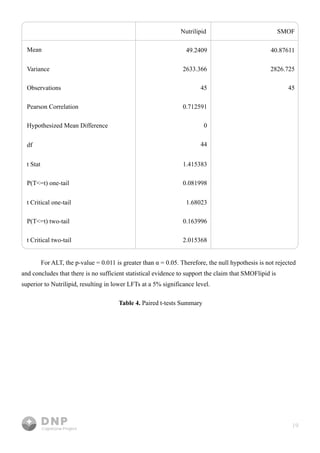
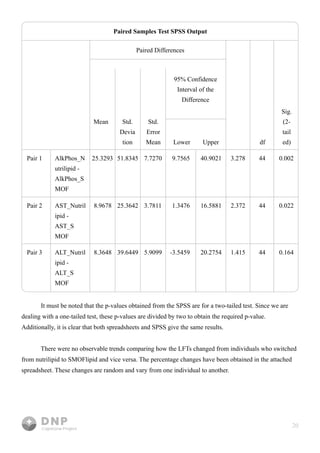
This study compares outcomes of lipid emulsions used in total parenteral nutrition (TPN) for home infusion patients, focusing on the efficacy of SMOFlipid versus NutriLipids. Results indicated that SMOFlipid led to lower liver function test levels for alkaline phosphate and AST, suggesting its superiority, although evidence for ALT was inconclusive. The research underscores the importance of effective lipid delivery in TPN and aims to improve treatment strategies for patients with malabsorption syndromes.