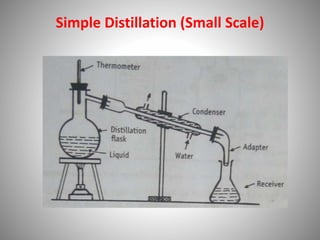
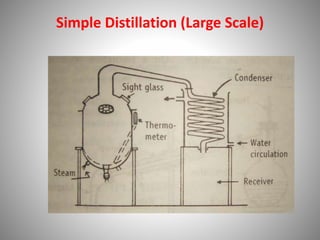
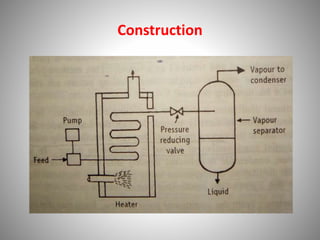
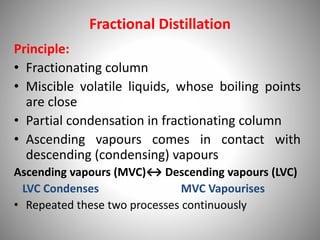
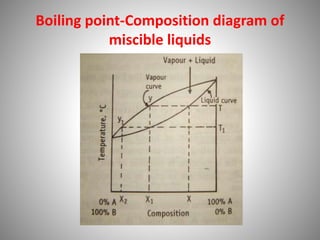
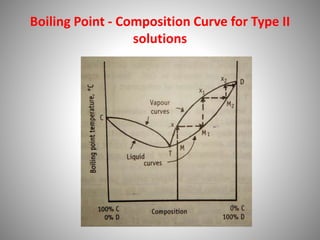
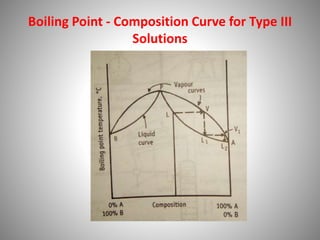
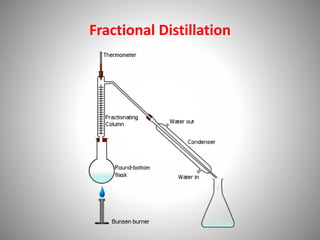
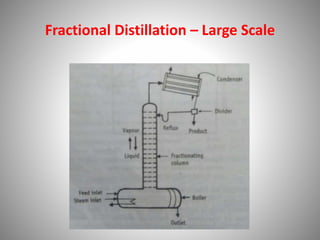
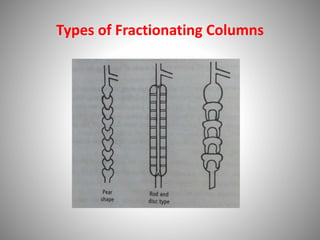
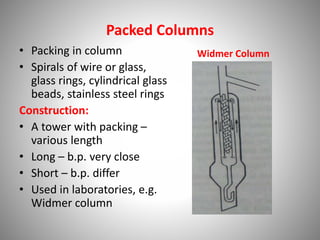
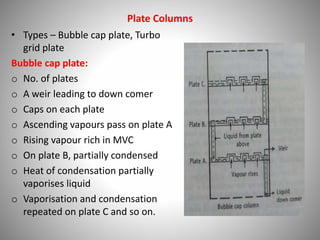
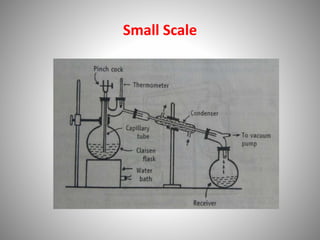
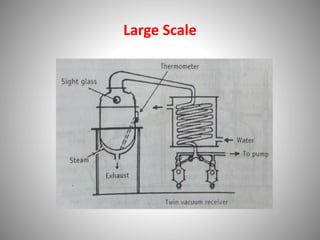
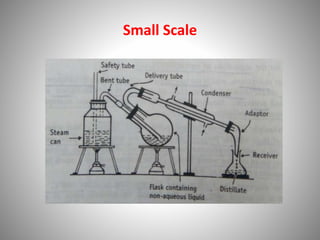
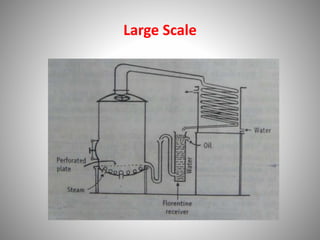
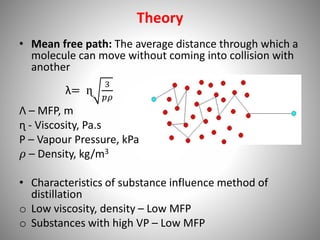
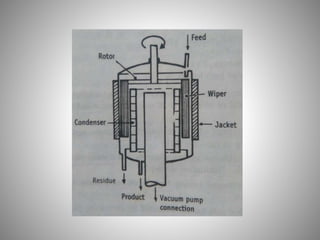
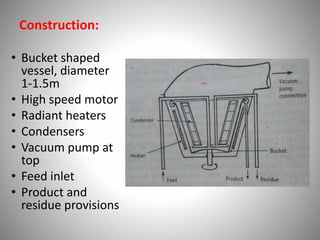
The document provides a comprehensive overview of distillation processes, including definitions, principles, and applications such as the separation of volatile oils and purification of organic solvents. It explains different types of distillation methods including simple, fractional, flash, and steam distillation, along with detailed descriptions of equipment and theoretical concepts involved like Raoult's law and azeotropic mixtures. Additionally, it highlights various types of distillation columns and techniques used in both small and large-scale setups.