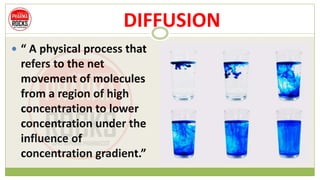
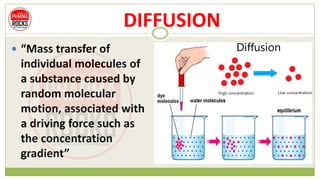
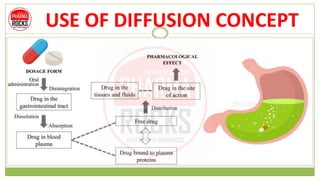
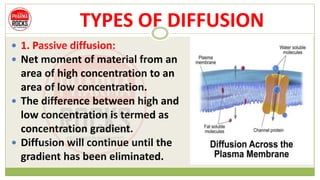
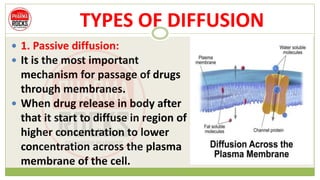
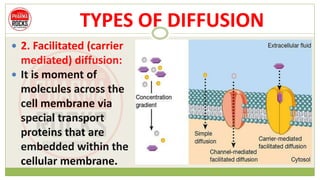
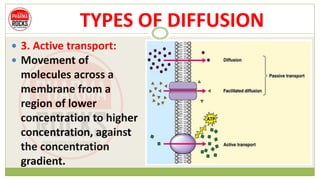
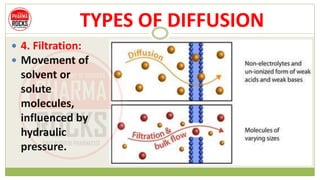
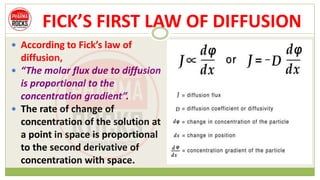
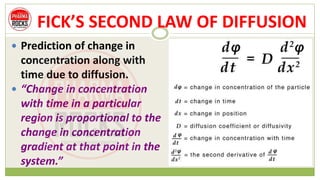
This document discusses diffusion principles that are important in biological systems and pharmaceutical sciences. It defines diffusion as the net movement of molecules from an area of high concentration to low concentration down a concentration gradient. The document outlines different types of diffusion including passive, facilitated, active, and filtration. It also describes Fick's Laws of Diffusion, which state that the rate of diffusion is proportional to the concentration gradient. Fick's laws are applied to understand processes like drug release from dosage forms and distribution in tissues.