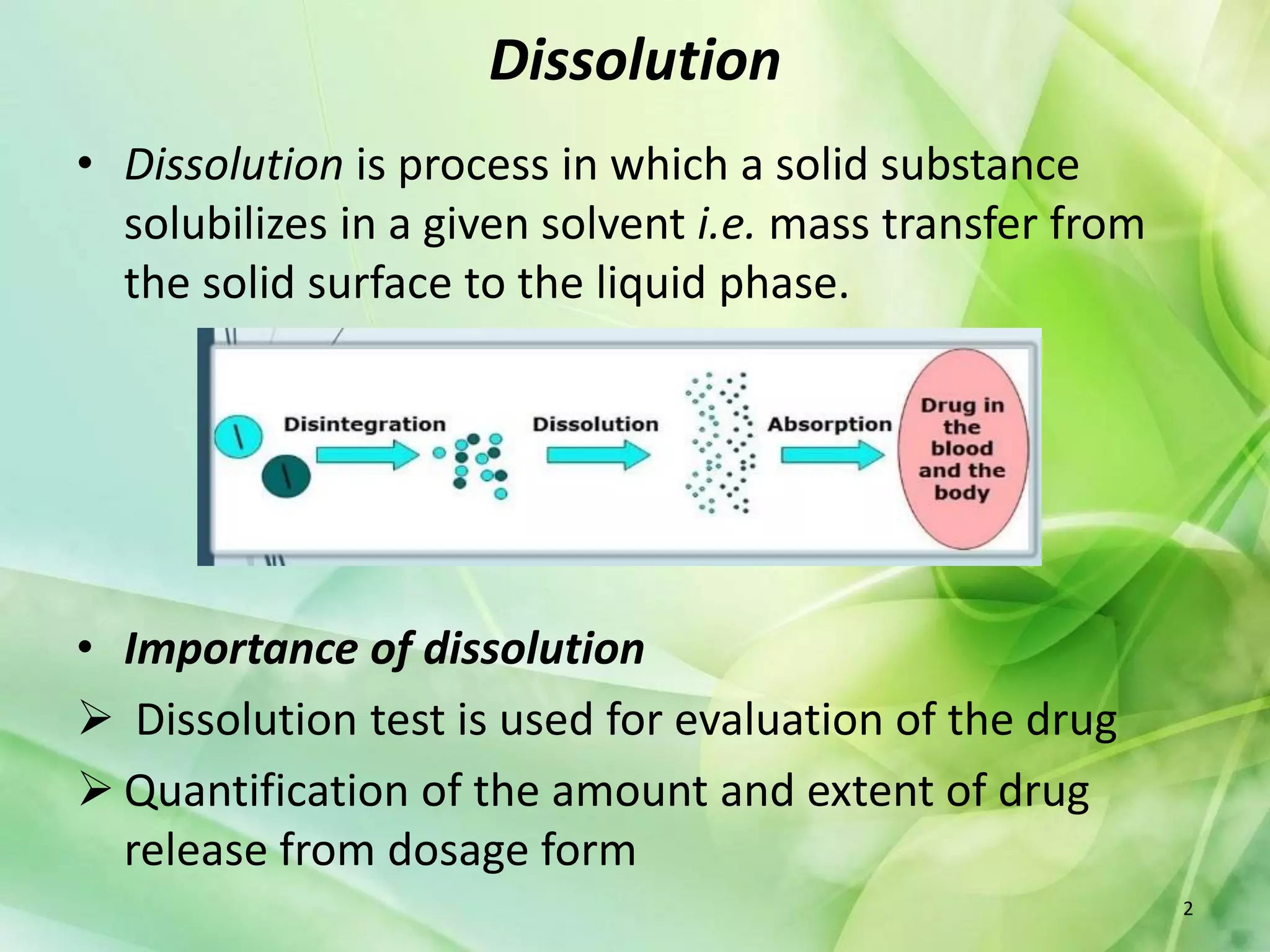
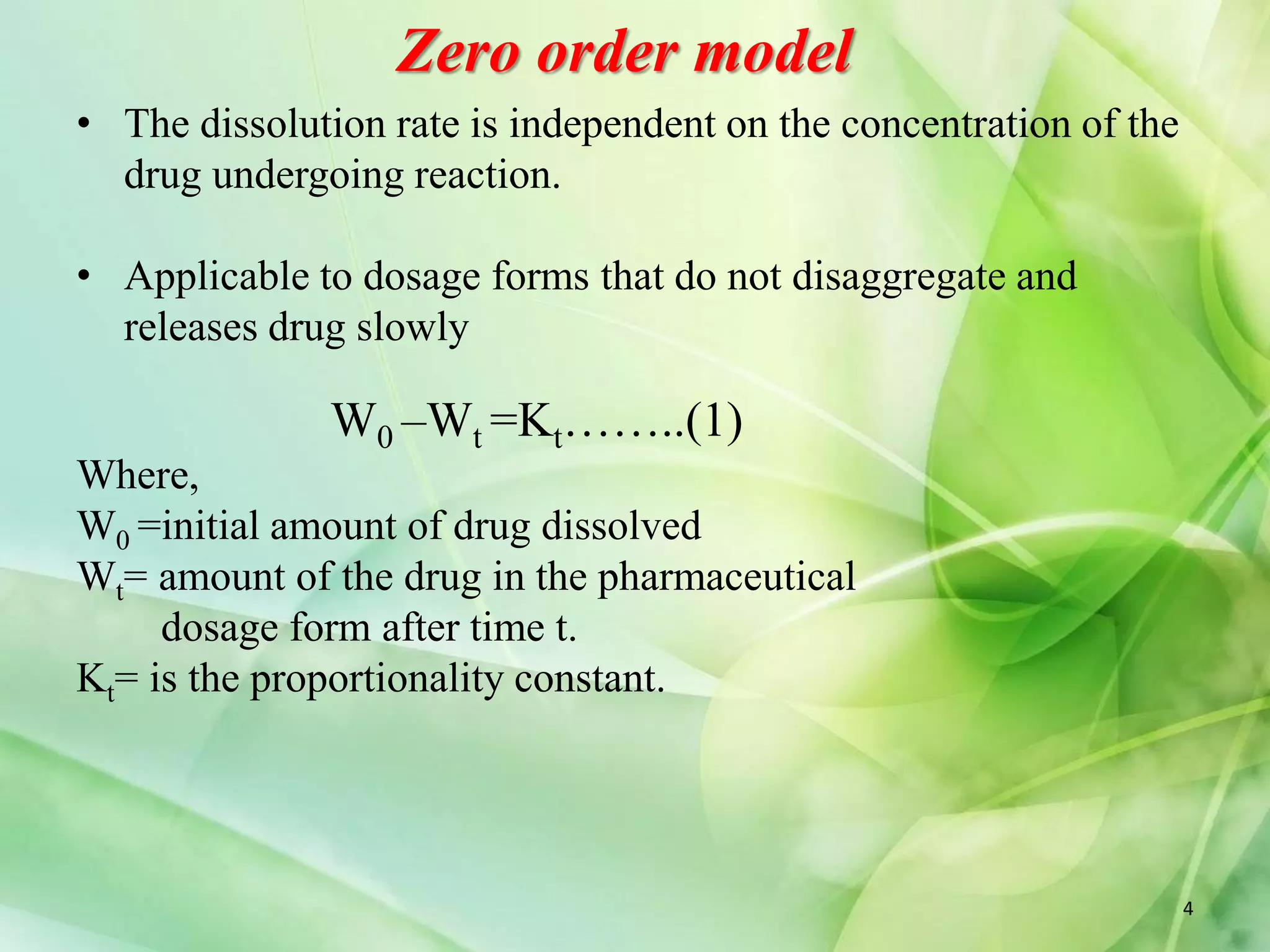
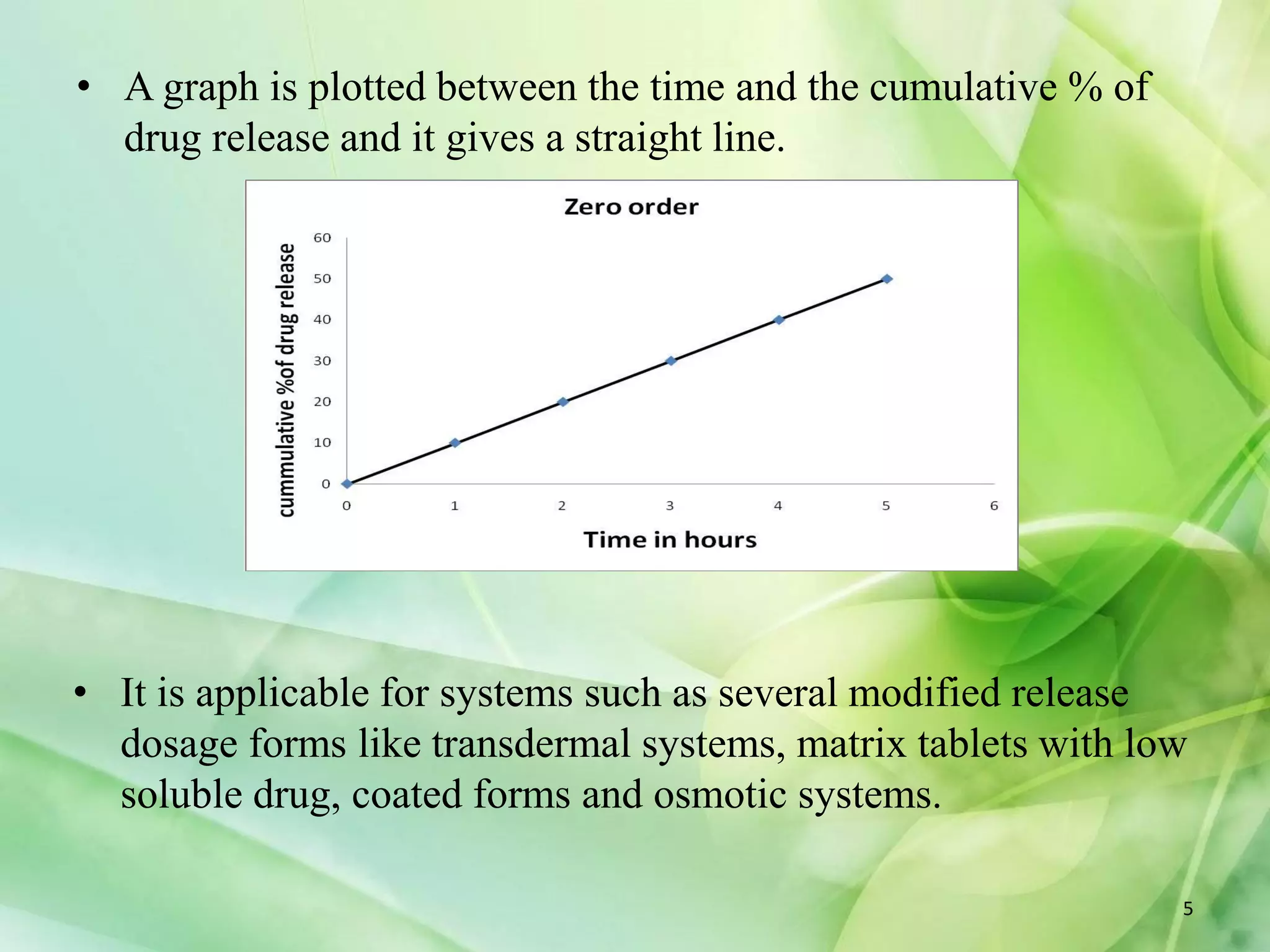
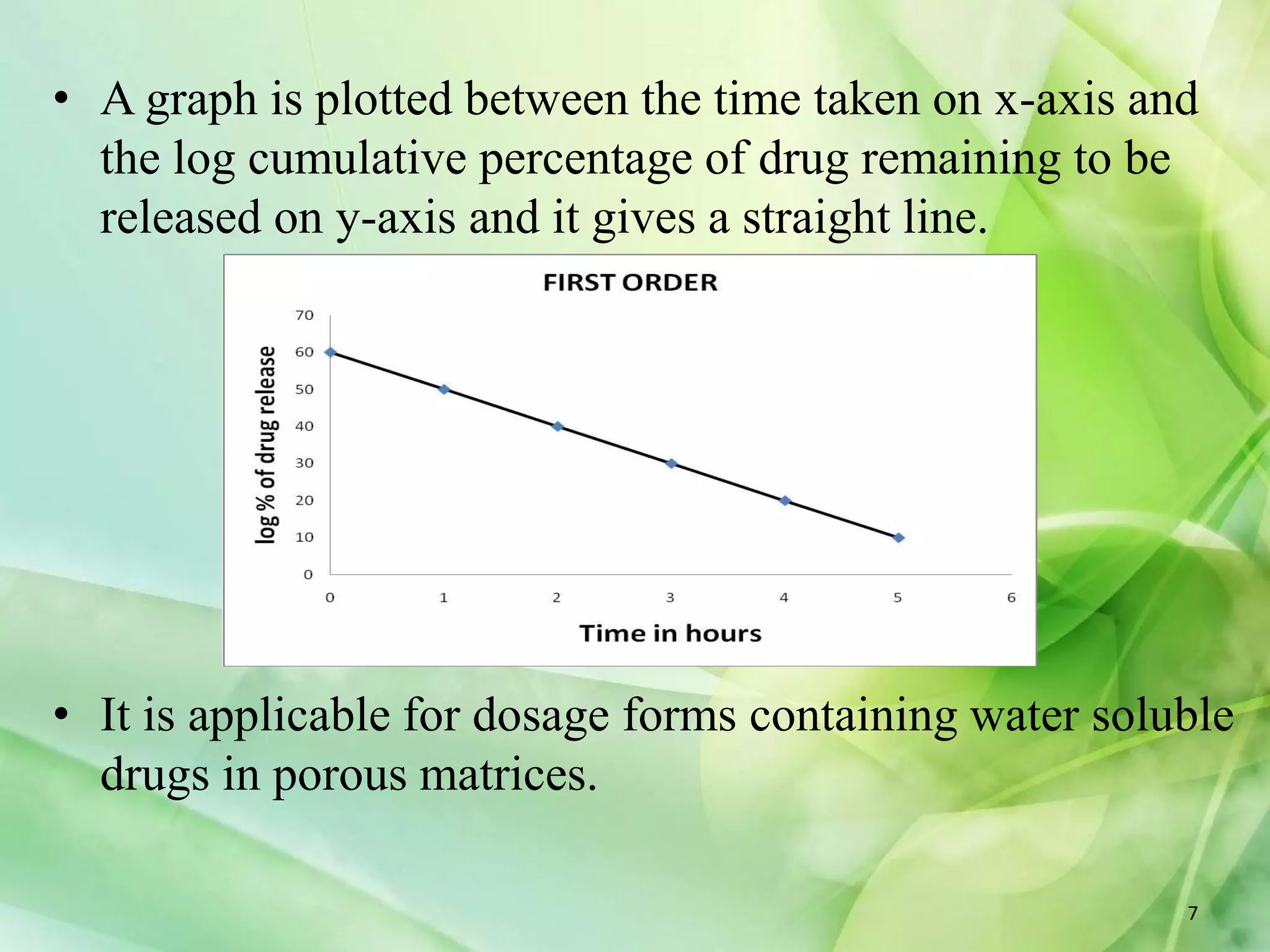
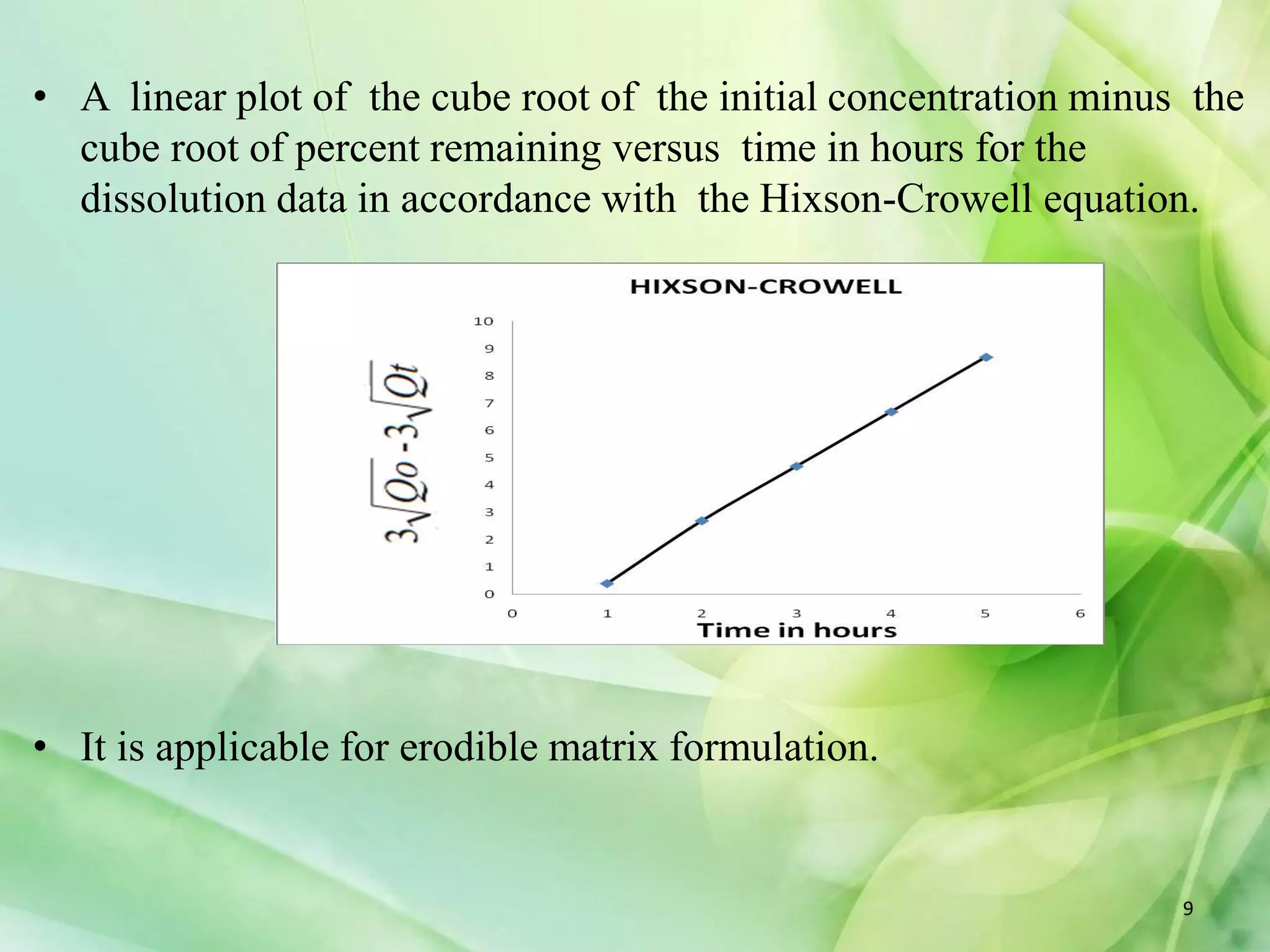
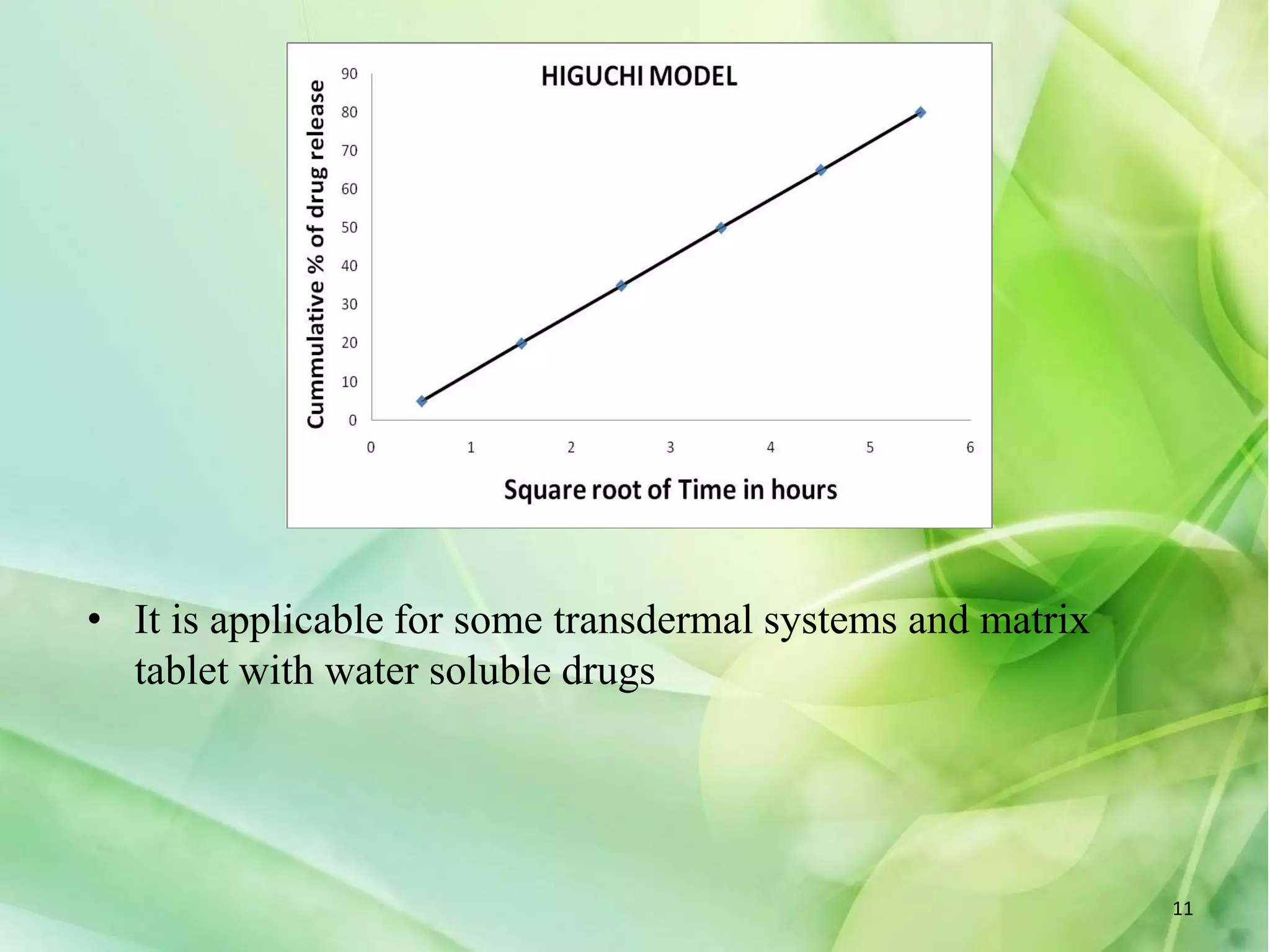
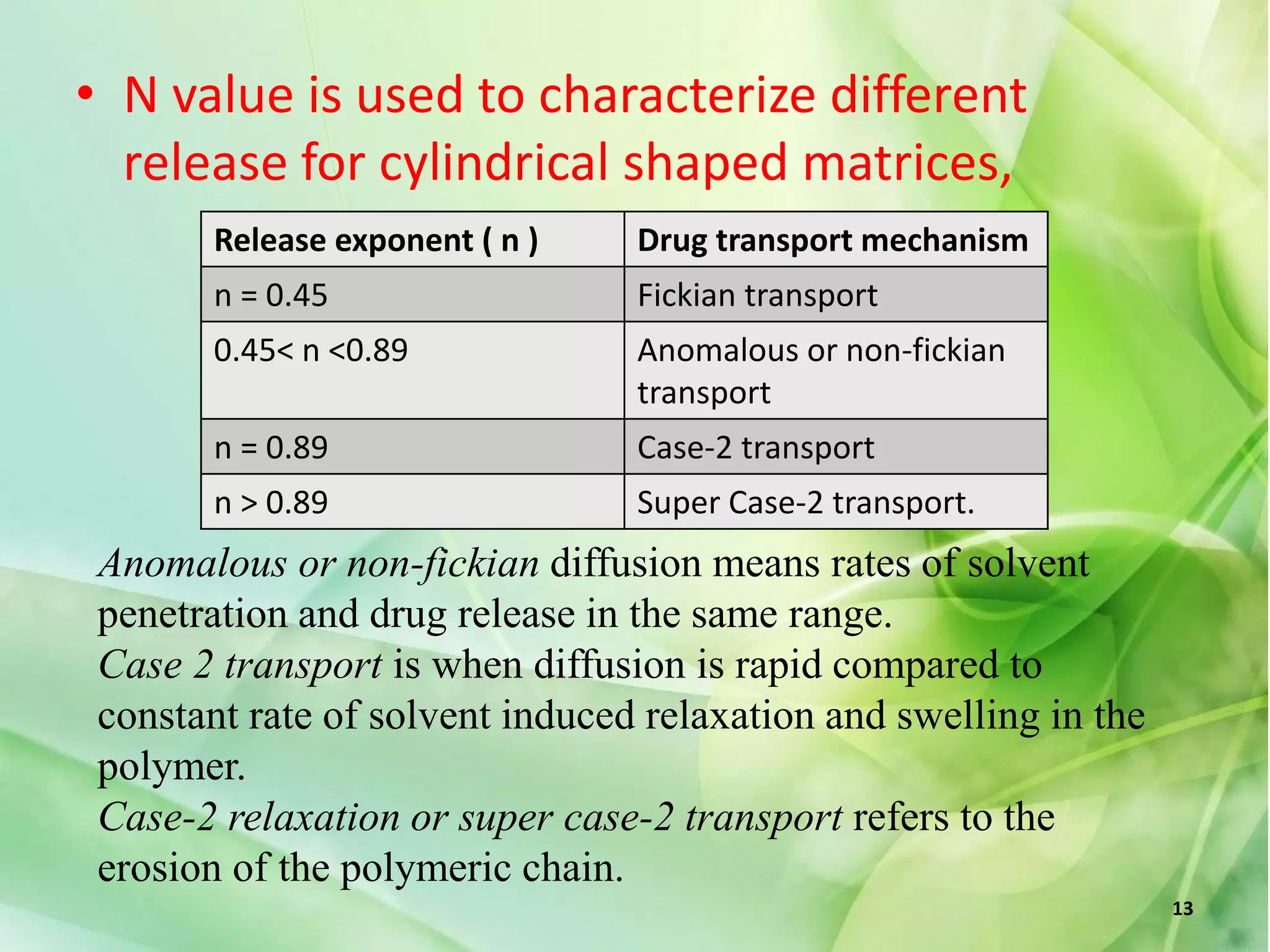
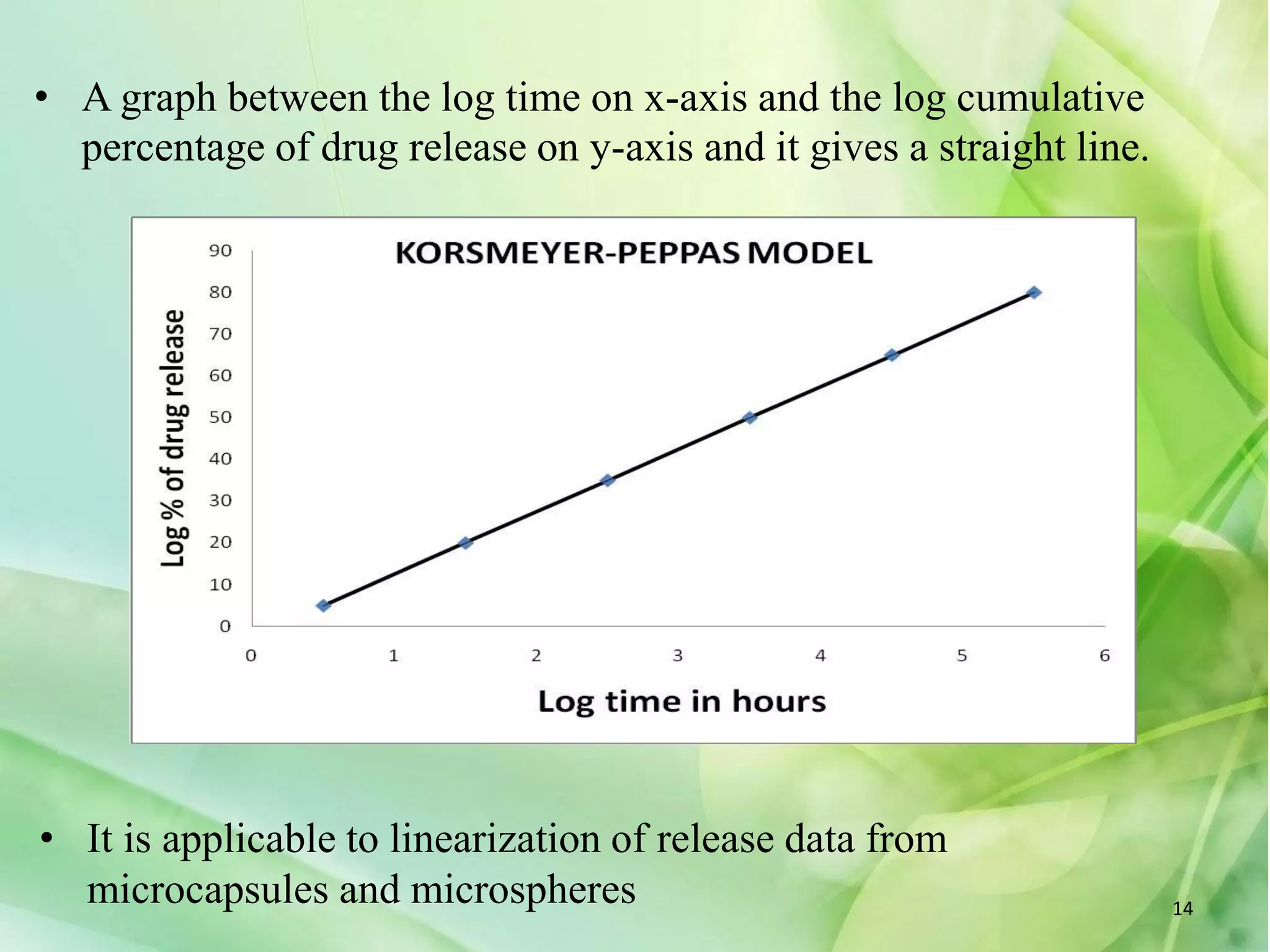
This document discusses various mathematical models that can be used to determine the kinetics of drug release from pharmaceutical dosage forms, including zero-order, first-order, Hixon-Crowell, Higuchi, Korsemeyer-Peppas, and Weibull models. It provides the key equations for each model and describes scenarios in which each model is applicable, such as matrix tablets, coated forms, transdermal systems, and others. The models can be used to analyze drug dissolution data and understand the mechanisms of drug release.