The document discusses guidelines for decontamination and decommissioning of pharmaceutical facilities. It covers:
1. The need to develop a comprehensive decommissioning project plan early involving all stakeholders.
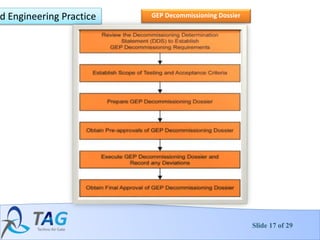
2. Regulatory requirements for decontamination, cleaning validation, risk assessment and closure documentation that must be met.
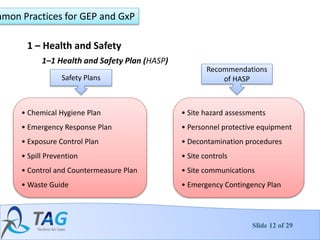
3. Common practices for decontamination including health and safety plans, training, exposure control and establishing decontamination zones.
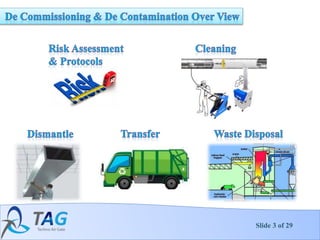
4. The decontamination and decommissioning process which involves phases like planning, initial assessment, remediation, demolition and final status surveys.