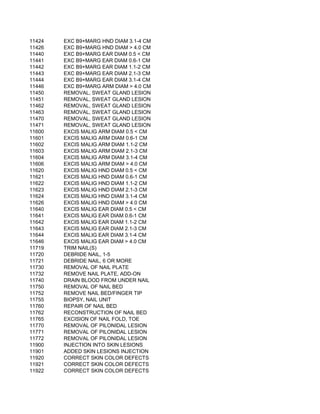
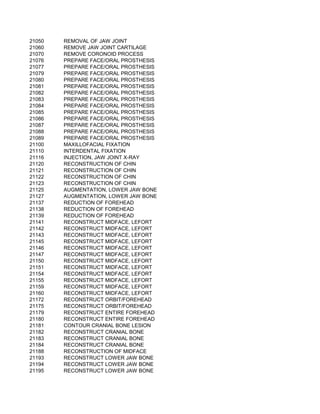
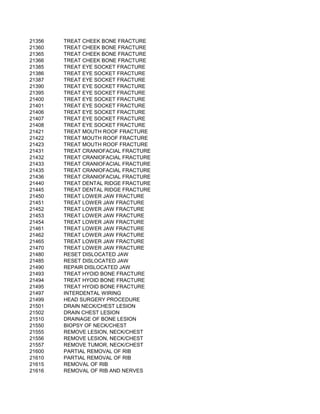
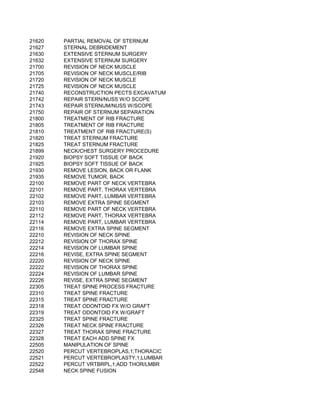
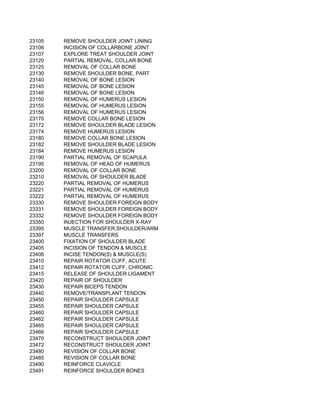
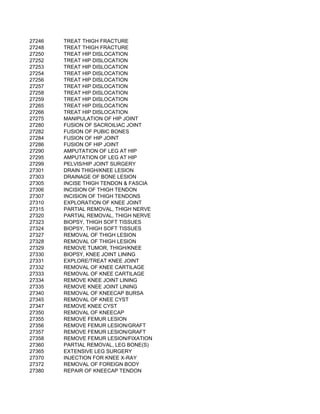
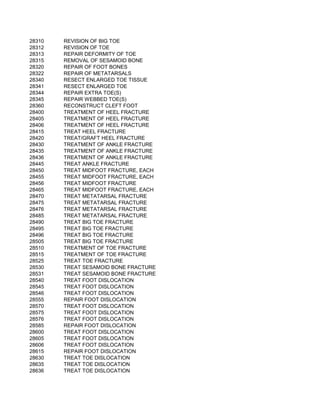
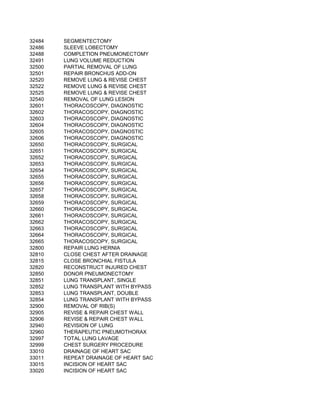
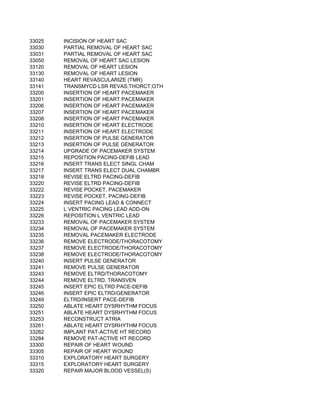
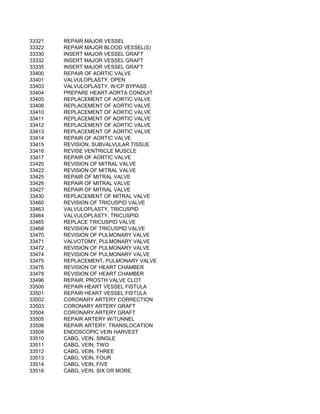
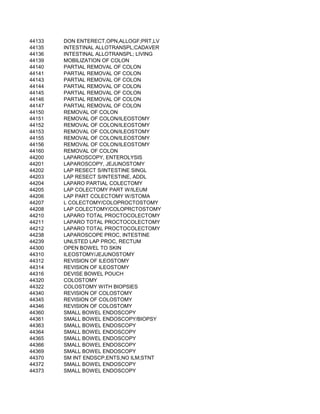
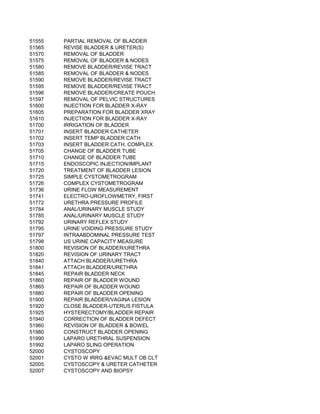
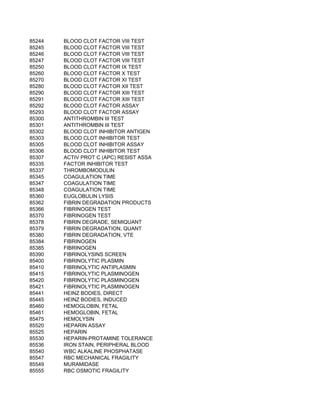
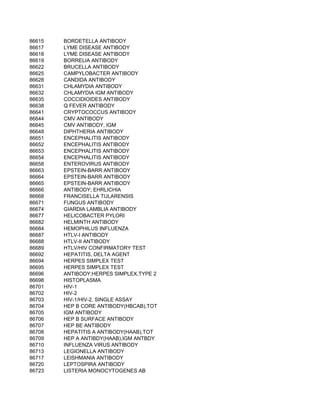
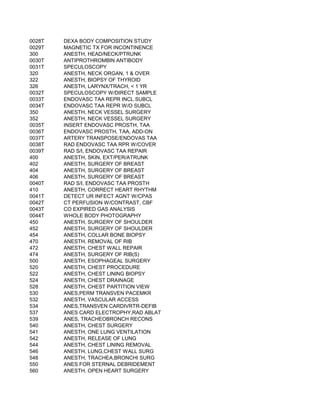
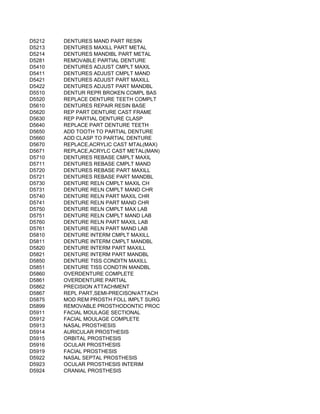
This document contains a list of over 300 CPT procedure codes ranging from 10021 to 27006. CPT codes are used in medical billing to describe medical, surgical, and diagnostic services and are between 3 to 5 numeric digits long. The codes cover a wide variety of medical specialties and services including dermatology, surgery, radiology, and more.










































































































































































































































































































































































































































































































































































































































































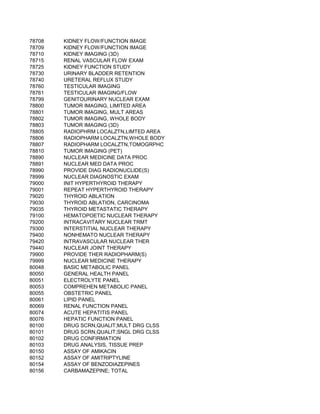
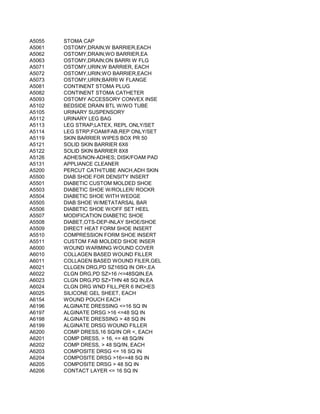
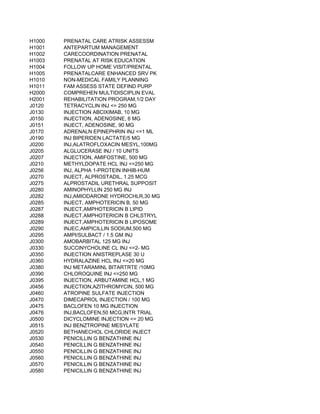
![57/58, PER 0.5 MICROCURIE
LINE, PER 0.5 MILLICURIE
E, PER 0.5 MILLICURIE
ARCITUMOMAB, PER VIAL
SODIUM GLUCOHEPTONATE, PER VIAL
SUCCIMER, PER VIAL
TONGUE SOMNOPLASTY COAGULATING ELECTRODE
SYSTEM, AND PAINBUSTER PAIN MANAGEMENT SYSTEM
SE F18 (2-DEOXY-2-[18F]FLUORO-D-GLUCOSE), PER DOSE (4-40 MCI/ML)
AST, ABDOMEN
REAST; UNILATERAL
REAST; BILATERAL
AST, CHEST (EXCLUDING MYOCARDIUM)](https://image.slidesharecdn.com/cptcodes13524/85/cpt_codes-1-653-320.jpg)






















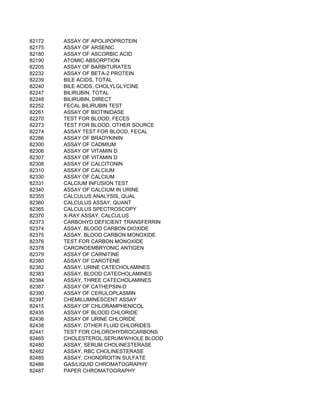
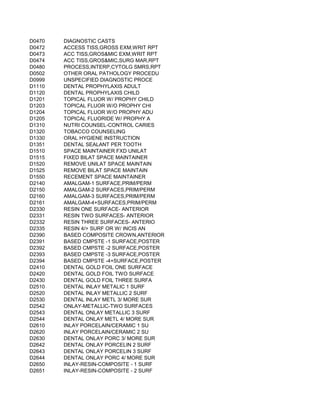
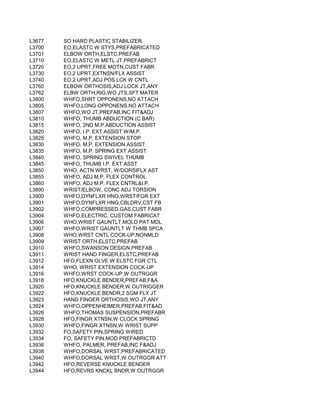
![NUT ES) [DEMO PROJECT CODE ONLY]
ENT PROGRAM
NCE OF ALCOHOL AND/OR DRUGS
N PROGRAM INPATIENT)
OGRAM INPATIENT)
N PROGRAM OUTPATIENT)
OGRAM OUTPATIENT)
NSE L;CRISIS INTERVENTN,& ACT THERAPIES/EDUC
LATORY SETTING)
HOUT ROOM AND BOARD, PER DIEM
NT PROGRAM), WITHOUT ROOM AND BOARD, PER DIEM
SIDENTIAL TREATMENT PROGRAM WHERE STAY IS TYPICA
VISION OF THE DRUG BY A LICENSED PROGRAM)
D BY PROVIDERS)
POPULATION)
ECT OR NON-DIRECT CONTACT WITH SERVICE AUDIENCES
ARGET POPULATION TO AFFECT KNOWLEDGE, ATTITUDE AN
OF SERVICES TO DEVELOP SKILLS OF IMPACTORS)
PR EVENTION THROUGH POLICY AND LAW)
TI NCLUDE ASSESSMENT
EV ENTS)
OTHER THAN BLOOD](https://image.slidesharecdn.com/cptcodes13524/85/cpt_codes-1-676-320.jpg)