CORD Rare Drug Timeline: Rare Drug Strategy Webinar
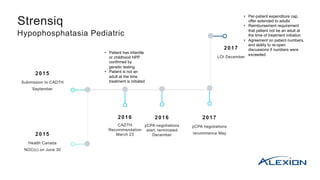
- 1. 2015 Submission to CADTH September 2015 Health Canada NOC(c) on June 30 2016 CADTH Recommendation March 23 2016 pCPA negotiations start; terminated December 2017 pCPA negotiations recommence May 2017 LOI December Strensiq Hypophosphatasia Pediatric • Patient has infantile or childhood HPP confirmed by genetic testing • Patient is not an adult at the time treatment is initiated • Per-patient expenditure cap, offer extended to adults • Reimbursement requirement that patient not be an adult at the time of treatment initiation • Agreement on patient numbers, and ability to re-open discussions if numbers were exceeded
- 2. 2005 CADTH Report (May) • 2nd Recommendation – DNL 2000 Submission to Health Canada 2003 USA Approval Apr 2004 Health Canada Approval Fabrazyme Fabry Disease Nov 2004 CADTH Submission Report • Recommendation – DNL Dec 2004 CADTH Resubmit
- 3. 2018 Health Canada Approval (Aug) Aug-Nov 2019 INESSS Submission (Aug) pCPA Submission (Nov) Dec 2019 Tegsedi hATTR neuropathy 2020 pCPA Decision (Apr) CADTH Submission • Report • Recommendation – Price reduction
- 4. 2018 Health Canada Approval (Jun) Sep 2019 INESSS Submission Nov 2019 pCPA Submission Onpattro hATTR neuropathy all stages Jun 2019 CADTH Submission • Report • Recommendation – Price Reduction Nov 2020 pCPA Decision
- 5. Health Canada Approval CADTH Submission • Recommendation – Price Reduction INESSS Submission - No pCPA Decision Vydaquel hATTR cardiomyopathy Feb 2020 Jun 2020 Feb 2021 pCPA Submission
- 6. 2013 Health Canada Approval (Mar) CADTH Report (Jul) • Recommendation – DNL 2015 Ontario: EAP critically ill excluding kidney transplant 2016 Ontario: include 6 months for kidney transplant; 02 2017 removed 6 months limit 2017 Most provinces some access but only Ontario clear criteria Soliris atypical hemolytic uremic syndrome (aHUS)
- 7. 2019 USA Approval (Oct) 2020 EMA Approval (Jun) Jan 2021 Health Canada Submission CADTH Submission Trikafta Cystic Fibrosis F508del mutation (90%) Jun 2021 Health Canada Approval • Indication – CF 12 years+ Nov 2021 CADTH Report • Recommendation – Price reduction Sep 2021 INESSS • w/ restrictive criteria pCPA Add to Kalydeco / Orkambi
- 8. 2019 USA Approval (May) 2020 Health Canada Approval (Dec) Indication – SMA < 20 kg Feb 2021 pCPA Submission Provincial Actions Zolgensma Spinal Muscular Atrophy (SMA) < 2 years • AB: Dec 2020 treat 2 yr+ infant; Feb 2021 2 yr; Dec 2021 AB RD program • Jan 2021 First Nation baby under federal Jordan's Principle • ON: 11-09-2020 Submission; 25-01-2021 Complete; Available thru EAP • ON: Newborn Screening pilot Jan 2020; 1st baby diagnosed Jan 2020; Spinraza 3 weeks; Zolgensma 5 weeks Oct 2021 pCPA Decision Mar 2021 CADTH Report • Recommendation – <180 days price reduction
- 9. 2017 USA Approval 2019 Health Canada Submission (Oct) Mar 2020 CADTH Submission 2021 pCPA • Submission (Nov) • Decision – Not concluded Luxturna RPE65 retinal dystrophy Oct 2020 Health Canada Approval • Indication – biallelic RPE65 mutations causing retinal dystrophy Nov 2020 CADTH Report • Recommendation – Price reduction Dec 2020 INESSS
- 10. Jan Feb Mar Apr May Jun Jul Aug Sep Oct Nov Dec 2017 Jan Feb Mar Apr May Jun Jul Aug Sep Oct Nov Dec Feb Mar Apr May Jan Jun 1st Submission 2018 2019 2016 Nov Dec 1. https://health-products.canada.ca/noc-ac/info.do?lang=en&no=19476 , https://hpr-rps.hres.ca/reg-content/regulatory-decision-summary-detail.php?lang=en&linkID=RDS00268 2. https://cadth.ca/nusinersen 3. https://cadth.ca/nusinersen-0 4. https://www.pcpacanada.ca/negotiation/21008 5. https://www.pcpacanada.ca/negotiation/21104 For 5q SMA children and adults Resubmission: 5q SMA across all types (including pre-symptomatic patients and all ages) • 5q SMA homozygous gene deletion, homozygous mutation, or compound heterozygote. • two copies of survival motor neuron 2 (SMN2) gene. • < 26 weeks disease; SMA symptoms 1st week to 7 months • Not currently requiring permanent invasive ventilation. < • are pre-symptomatic with two or three copies of SMN2, or • disease duration < six months, 2 copies SMN2, symptom onset 1st week -7 months • ≤ 12 yrs w/ymptom onset < 6 months, and never walked independently
- 11. 2015 Final Report August • ≥ six yrs • G551D mutation 2011-12 Pre-Submission Dec 2011 Priority Status: March 2012 HC Submission April 2012 2012 NOC November • ≥ 12 mos7 to 25 kg • CFTR gene mutations G551D, G1244E, G1349D, G178R, G551S, S1251N, S1255P, S549N or S549R • ≥ 6 yrs; ≤ 25 kg • CFTR gene mutation G551D, G1244E, G1349D, G178R, G551S, S1251N, S1255P, S549N or S549R • ≥ 18 yrs w/R117H mutation 2014 CADTH Submit May • CFC submit for additional mutations July • CADTH Recommendation December 2019 pCPA completed price negotiations on additional mutations PRESENT Request fast-track additional mutations Kalydeco Cystic Fibrosis CFC Advocacy on Additional Mutations
- 12. • Priority Status January • HC Submission January • NOC w/Safety: September • Acceptance Letter October 2015 • NOC January • improves lung function, reduces lung flare-ups, • two copies most common mutation F508del. 2016 • CADTH January • CDEC Recommendation October • Final Report April • (LUM/IVA) not be reimbursed in patients ≥12 years homozygous F508del mutation 2016 • CADTH February • CDEC Recommendation October • Final Report October • ORKAMBI® not be reimbursed by federal, provincial and territorial drug programs. 2018 • INESSS Review • Not recommend ORKAMBI® for public coverage, PRESENT Orkambi Cystic Fibrosis
- 13. 2018 2019 2020 2020 2020 PRESENT Health Canada Submission: May Approval: December Active Negotiation CADTH/INESSS Submissions: July pCPA Letter of Engagement: October CADTH: Approval (Initial) December treatment of X-linked hypophosphataemia (XLH) in adult and pediatric patients ≥ 1 year of age INESSS Notice to Minister: March 2020 CADTH Recommendation: May, 2020 (Final) • patients ≥ 1 year; no epiphyseal closure; • fasting hypophosphatemia, • normal renal function • Total rickets severity score (RSS) ≥ two • PHEX gene variant patient or direct family member • Not children w/XLH • Not adults w/XLH • If PLA: PHEX mutation patient or direct family • > normal FGF23 • Rickets Severity Score (RSS) ≥ 2; and • 12 mos. to 18 years • open growth plates. • Private Drug Coverage Compassionate Access Crysvita X-Linked Hypophosphataemia
- 14. FEBURARY 2021 Health Canada approval for DOJOLVI™ JULY 2020 Health Canada Submission Q3 2020 Q4 2020 Q1 2021 Q2 2021 Q3 2021 Q4 2021 Q1 2022 FEBRUARY 2022 Final CADTH Recommendation “Reimburse with Conditions” SEPTEMBER 2021 Draft CADTH Recommendation“ Do Not Reimburse” MARCH 2021 CADTH Submission CADTH Reconsideration Process JUNE 2020 FDA approval for DOJOLVI® Final CADTH Recommendation: • Reimburse for the treatment of adult and pediatric patients with acute life-threatening LC-FAOD who require alternative therapy to conventional even-chain MCT Note: Initiation of therapy and assessment of continued benefit should be reviewed by a panel of metabolic disease specialists Dojolvi Long-Chain Fatty Acid Oxidation Disorders
