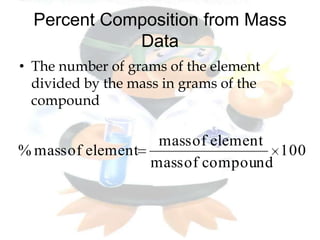
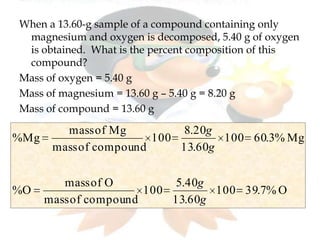
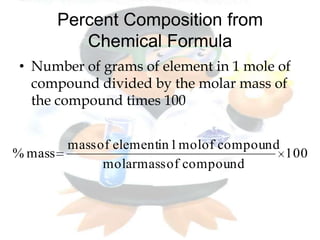
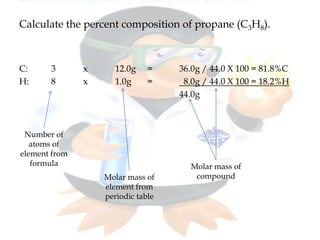
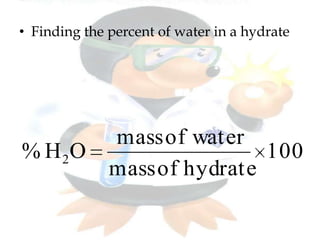This document discusses composition stoichiometry and chemical formulas. It explains the laws of definite and multiple proportions, and how they relate elements in compounds. It then covers calculating percent composition from mass data or chemical formulas, including for hydrates that contain a specific number of water molecules. Percent composition represents the percent by mass of each element in a compound, and can identify the elements and their amounts in an unknown compound.