This document discusses determining chemical formulas, including:
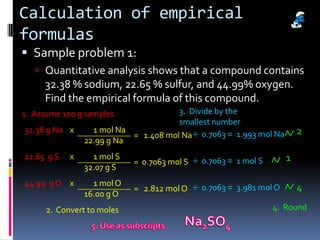
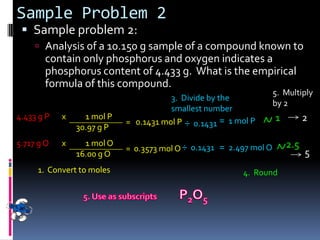
1) Defining empirical and molecular formulas, and how to calculate empirical formulas from percentage or mass composition data.
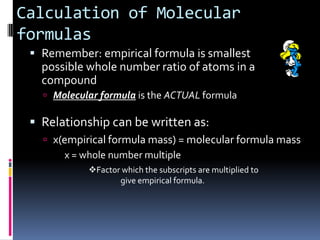
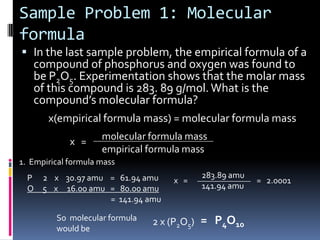
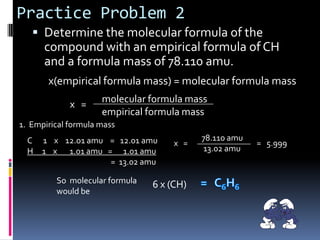
2) Explaining how to determine a molecular formula from an empirical formula using the relationship between molecular formula mass and empirical formula mass.
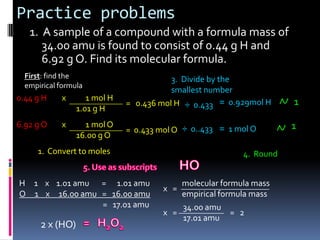
3) Providing examples of calculating empirical formulas from composition data and determining molecular formulas from empirical formulas and molar masses.