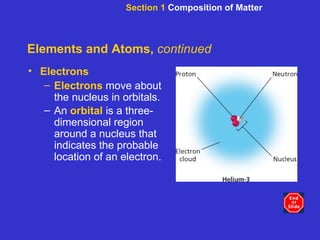
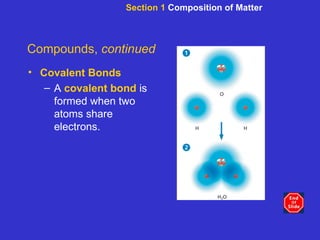
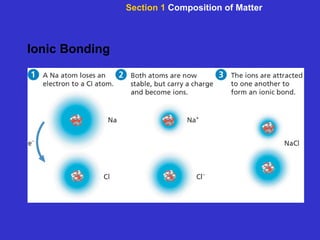
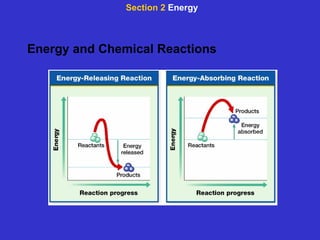
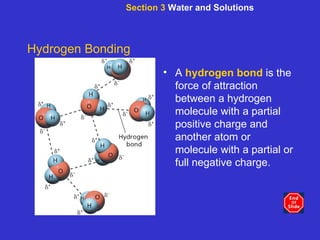
The document is an outline for a chemistry textbook covering topics like the composition of matter, energy, and water and solutions. It defines key terms like elements, atoms, isotopes, compounds, and bonds. It describes states of matter and energy changes during chemical reactions. It also explains the polarity and hydrogen bonding of water molecules and how this relates to properties like solubility, cohesion, adhesion and temperature moderation. Finally, it covers acids, bases, pH and buffers.