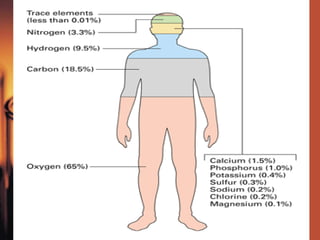
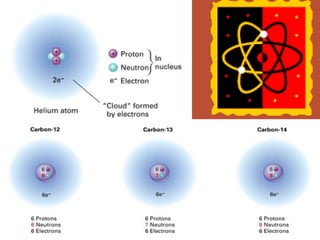
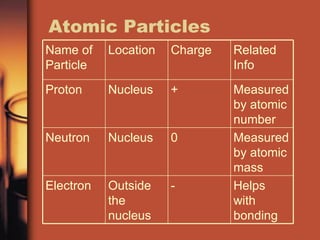
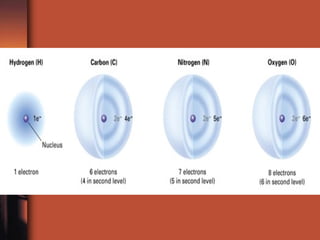
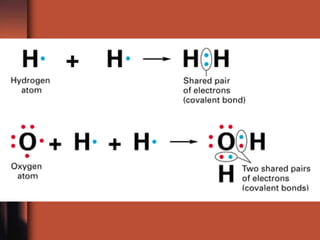
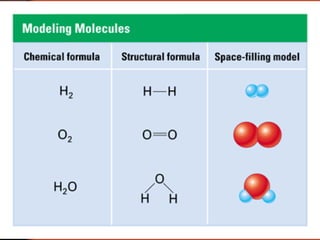
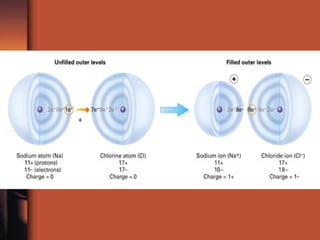
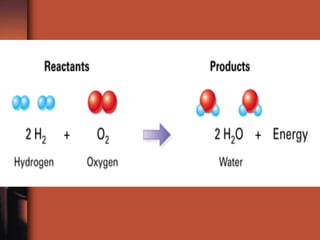
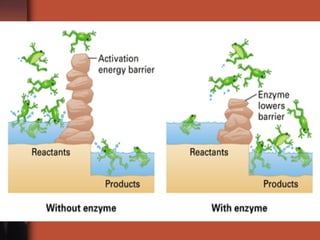
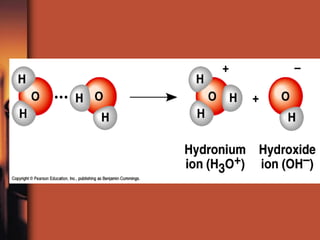
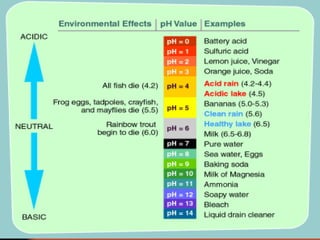
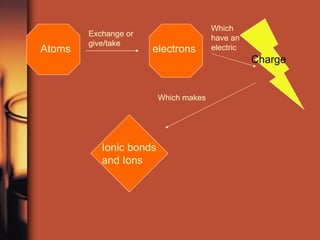
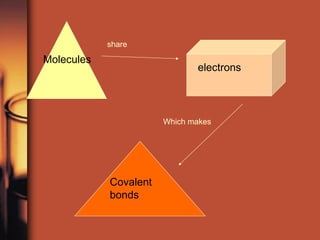
The document introduces basic chemistry concepts such as the composition of matter, elements and their symbols, atomic structure, bonds between atoms including ionic and covalent, states of matter, chemical reactions and energy, and acid-base solutions including pH and buffers. It defines terms like elements, atoms, protons, neutrons, electrons, compounds, molecules, and provides examples of chemical reactions and bonds between different types of atoms.