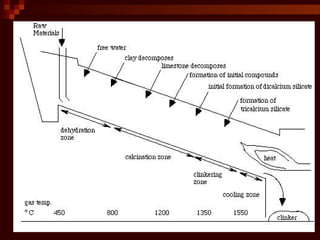
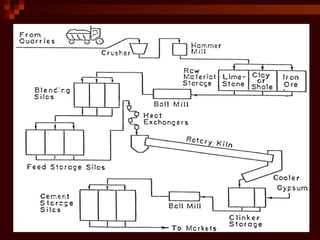
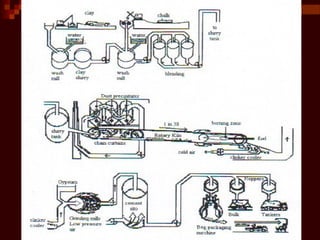
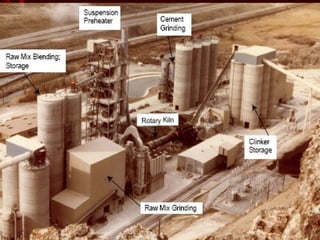
Cement is produced by heating limestone and clay at high temperatures. This causes them to chemically combine and form small balls called clinker. Clinker is then ground with gypsum into a powder to create cement. When mixed with water, cement forms a paste that binds sand, gravel and crushed rock together to form concrete. The key steps in cement production are grinding raw materials, firing the mixture in a kiln at over 1300°C to produce clinker, cooling the clinker, and grinding it with gypsum into the final cement powder. Different types of cement are produced by varying the chemical composition and fineness to achieve specific properties like rapid setting, low heat generation, or sulfate resistance.